https://goodmenproject.com/business-ethics-2/guys-saving-world-social-entrepreneurship-kldg/





Part II of the Bioquark Inc. show on Grognostics — https://www.stitcher.com/podcast/grognostics/e/53166919?autoplay=true

Rudrapur, Uttrakhand, India — July 02, 2017
Revita Life Sciences, (http://revitalife.co.in) a biotechnology company focused on translational regenerative therapeutic applications, has announced that it is continuing to advance their novel, multi-modality clinical intervention in the state of brain death in humans.
“We have proactively continued to advance our multi-modality protocol, as an extended treatment before extubation, in an attempt to reverse the state of brain death” said Mr.Pranjal Agrawal, CEO Revita Life Sciences. “This treatment approach has yielded some very encouraging initial outcome signs, ranging from minor observations on blood pressure changes with response to painful stimuli, to eye opening and finger movements, with corresponding transient to permanent reversal changes in EEG patterns.”
This first exploratory study, entitled “Non-randomized, Open-labelled, Interventional, Single Group, and Proof of Concept Study with Multi-modality Approach in Cases of Brain Death Due to Traumatic Brain Injury Having Diffuse Axonal Injury” is ongoing at Anupam Hospital, Rudrapur, Uttrakhand. The intervention primarily involves intrathecal administration of minimal manipulated (processed at point of care) autologous stem cells derived from patient’s fat and bone marrow twice a week.
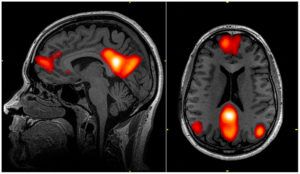
This study was inappropriately removed from the Indian Council of Medical Research (ICMR) database. ICMR has no regulatory oversight on such research in India.
The Central Drugs Standard Control Organization (CDSCO), Drug Controller General of India, had no objection to the program progressing. Regulatory approval as needed for new drugs, is currently not required when research is conducted on the recently deceased, although IRB and family consent is definitely required. CDSCO, the regulator of such studies, clearly states that “no regulatory requirements are needed for any study with minimal manipulated autologous stem cells in brain death subjects”.
Death is defined as the termination of all biological functions that sustain a living organism. Brain death, the complete and irreversible loss of brain function (including involuntary activity necessary to sustain life) as defined in the 1968 report of the Ad Hoc Committee of the Harvard Medical School, is the legal definition of human death in most countries around the world. Either directly through trauma, or indirectly through secondary disease indications, brain death is the final pathological state that over 60 million people globally transfer through each year.
“We are in process of publishing our initial retrospective results, as well ongoing early results, in a peer reviewed journal. These initial findings will prove invaluable to the future evolution of the program, as well as in progressing the development multi-modality regenerative therapeutics for the full range of the severe disorders of consciousness, including coma, PVS, the minimally conscious state, and a range of other degenerative CNS conditions in humans,” said Dr. Himanshu Bansal, Chief Scientific Officer, Revita Life Sciences and Director of Mother Cell.
With the maturation of the tools of medical science in the 21st century, especially cell therapies and regenerative medicines, tissues once considered irretrievable, may finally be able to be revived or rejuvenated. Hence many scientists believe that brain death, as presently defined, may one day be reversed. While the very long term goal is to find a solution for “re-infusing life”, the short term purpose of these types of studies is much less dramatic, which is to confirm if the current definition of brain irreversibility still holds true. There have been many anecdotal reports of brain death reversal across the world over the past decades in the scientific literature. Studies of this nature serve to verify and establish this very fact in a scientific and controlled manner. It will also one day give a fair chance to individuals, who are declared brain dead, especially after trauma.
About Revita Life Sciences
Revita Life Sciences is a biotechnology company focused on the development of stem cell therapies and regenerative medicine interventions that target areas of significant unmet medical need. Revita is led by Dr. Himanshu Bansal MD, who has spent over two decades developing novel MRI based classifications of spinal cord injuries as well as comprehensive treatment protocols with autologous tissues including bone marrow stem cells, Dural nerve grafts, nasal olfactory tissues, and omental transposition.
Philadelphia, PA, USA / Moscow, Russia — Bioquark, Inc., (http://www.bioquark.com) a life sciences company focused on the development of novel bio-products for regeneration, disease reversion, and healthy aging, announced the commercial approval of naturally derived Bioquantine food ingredients in the Eurasian Customs Union (formerly known as the Customs Union of Belarus, Kazakhstan, and Russia). Moscow based, Lakmus LLC, a diversified investment company with business interests in pharmacies, restaurants, and real estate, collaborated with Bioquark Inc. on the regulatory approvals.
“We are very excited about this successful regulatory approval,” said Ira S. Pastor, CEO, Bioquark Inc. “The commercialization of Bioquantine food ingredients, including functional foods, drinks, and dietary supplements, represents another important step in our continued evolution as a company focused on a broad range of products and services in the regenerative healthcare space.”
Throughout the 20th century, natural products formed the basis for a majority of all pharmaceuticals, biologics, and consumer healthcare products used by patients around the globe, generating trillions of dollars of wealth. However, many scientists believe we have only touched the surface with what the natural world, and its range of organisms, which from a health and wellness perspective are much further advanced than human beings, has to teach us.
The integration of a complex set of newer research disciplines, including interkingdom signaling, semiochemical communication, and evolutionary biology, as well as significant recent activity in the areas of the microbiome and virome, are highlighting a myriad of new ways that non-human bio-products can affect the human genome for positive transitions in health and wellness dynamics.
“Bioquark has spent several years studying the natural ability of many species to turn back biological time in order to maintain health, fitness, and survival,” said Dr. Sergei Paylian, Founder, CSO, and President, Bioquark Inc. “This Eurasian initiative is one more step in the path in allowing humans to recapture these capabilities to effectively counter our unfortunate progression into aging, disease and degeneration.”
About Bioquark, Inc.
Bioquark Inc. is focused on the development of natural biologic based products, services, and technologies, with the goal of curing a wide range of diseases, as well as effecting complex regeneration. Bioquark is developing both biological pharmaceutical candidates, as well as products for the global consumer health and wellness market segments.
Link to Press — http://www.prweb.com/releases/2017/01/prweb13955162.htm

Bioquark Inc. (www.bioquark.com) Interview in MoneyWeek
Read whole story: http://moneyweek.com/who-wants-to-live-forever/

Philadelphia, PA, USA / Mexico City, Mexico — Bioquark, Inc., (www.bioquark.com) a life sciences company focused on the development of novel bioproducts for complex regeneration, disease reversion, and aging, and RegenerAge SAPI de CV, (www.regenerage.clinic/en/) a clinical company focused on translational therapeutic applications of a range of regenerative and rejuvenation healthcare interventions, have announced a collaboration to focus on novel combinatorial approaches in human disease and wellness. SGR-Especializada (http://www.sgr-especializada.com/), regulatory experts in the Latin American healthcare market, assisted in the relationship.
“We are very excited about this collaboration with RegenerAge SAPI de CV,” said Ira S. Pastor, CEO, Bioquark Inc. “The natural synergy of our cellular and biologic to applications of regenerative and rejuvenative medicine will make for novel and transformational opportunities in a range of degenerative disorders.”
As we close in on $7 trillion in total annual health care expenditures around the globe ($1 trillion spent on pharmaceutical products; $200 billion on new R&D), we are simultaneously witnessing a paradoxical rise in the prevalence of all chronic degenerative diseases responsible for human suffering and death.
With the emergence of such trends including: personalization of medicine on an “n-of-1” basis, adaptive clinical design, globalization of health care training, compassionate use legislative initiatives for experimental therapies, wider acceptance of complementary medical technologies, and the growth of international medical travel, patients and clinicians are more than ever before, exploring the ability to access the therapies of tomorrow, today.

The estimate of the current market size for procedural medical travel, defined by medical travelers who travel across international borders for the purpose of receiving medical care, is in the range of US $40–55 billion.
Additionally, major clinical trial gaps currently exist across all therapeutic segments that are responsible for human suffering and death. Cancer is one prime example. As a leading cause of morbidity and mortality worldwide for many decades, today there are approximately 14 million new cases diagnosed each year, with over 8 million cancer related deaths annually. It is estimated that less than 5% of these patients, take the initiative to participate in any available clinical studies.
“We look forward to working closely with Bioquark Inc. on this exciting initiative,” said Dr. Joel Osorio, Chief of Clinical Development RegenerAge SAPI de CV. “The ability to merge cellular and biologic approaches represents the next step in achieving comprehensive regeneration and disease reversion events in a range of chronic diseases responsible for human suffering and death.”

About Bioquark, Inc.
Bioquark Inc. is focused on the development of natural biologic based products, services, and technologies, with the goal of curing a wide range of diseases, as well as effecting complex regeneration. Bioquark is developing both biological pharmaceutical candidates, as well as products for the global consumer health and wellness market segments.
About RegenerAge SAPI de CV
RegenerAge SAPI de CV is a novel clinical company focused on translational therapeutic applications, as well as expedited, experimental access for “no option” patients, to a novel range of regenerative and reparative biomedical products and services, with the goal of reducing human degeneration, suffering, and death.