https://www.bustle.com/p/7-creepy-things-a-dead-body-can-do-according-to-science-13550864




The Alzheimer’s Hypothesis
Introduction
Alzheimer’s disease was first discovered in 1907 in a 51 year old woman by the German physician A. Alzheimer. One of the first changes noticed was an eruption of jealous feelings towards her husband. It wasn’t long before symptoms of rapid memory impairment were observed. The impairments prevented her from finding her way out of her home, She hid herself, she would drag objects to and fro, and occasionally screamed because she believed people were out to kill her.
When she was institutionalized her gestures would show a complete helplessness. As common in most Alzheimer’s patients, she was disoriented as to time and place. At times she would state that she didn’t understand anything, felt confused, and totally lost. When the doctor came in to see her she would consider it as an official visit and would apologize for not having finished her work. Other times she would be terrified and start to yell that the doctor wanted to operate on her. Other times she would send him away in complete indignation uttering phrases indicating that she was afraid that the doctor wanted to damage her woman’s honor. At times she would become completely delirious , dragging her blankets and to and fro, calling for her husband and daughter, and seeming to experience auditory hallucinations. She would often scream for hours and hours in a horrible voice. Mental regression advanced quite steadily. After four and a half years of illness the patient finally died.
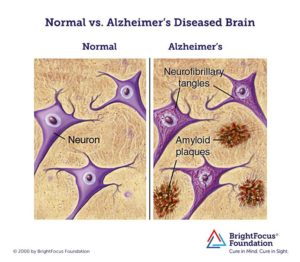
Alzheimer performed a postmortem examination of the woman’s brain. He paid special attention to changes in the “neurofibrils,” fibers in the cytoplasm of a nerve axon — elements of the cytoskeleton that can be stained by a silver solution.
The Bielschowsky silver preparation showed very characteristic changes in the neurofibrils. Looking inside an apparently normal-looking cell, however, one or more single fibers became observably prominent through their striking thickness and specific impregnability. At a more advanced stage many many fibers arranged in parallel showed the same changes. Over time they formed dense bundles and gradually advanced to the surface of the cell. The cell and nucleus would eventually disappear, leaving only a tangled bindle of fibrils where the neuron once was.
Since these fibrils can be readily stained with certain dyes, a chemical transformation of the fibril substance must have taken place. This might be why the fibrils survived the destruction of the cell. The transformation of the fibrils seems to go hand in hand with the storage of an as-of-yet not closely investigated pathological byproduct of neuronal metabolism. Such alterations were found in about one-quarter to one-third of all the neurons in the cerebral cortex. Numerous neurons, especially in the upper cell layers, had completely disappeared.
The number and distribution of neurofibrillary tangles, what are commonly known as the “tombstones” of dead and dying neurons, are correlated with the severity of the symptoms in Alzheimer’s disease.
Phase1
What is the purpose of beta-amyloid?
Alzheimer’s disease causes impairment with memory, thinking, and behavior. Alzheimer’s affects 5.5 million people age 65 and older and approximately 200,000 individuals under the age 65. Alzheimer’s disease is the 5th leading cause of death in the United States. The root cause of Alzheimer’s is still unknown. There are several hypothesis that attempt to explain it. This article focuses on the research being done in a joint effort between a team from Harvard University being led by Rudolf Tanzi and Robert Moir at Boston Massachusetts General Hospital.
Tanzi and Moir investigated the development of Alzheimer’s disease in animals and found the development of amyloid plaques is not unique to humans. 70 percent of vertebrates share certain sequences of particular amino acids in human beta-amyloid. Moir believes because this sequence is shared so widely and has not changed over time that it must be doing something very important. Moir discovered that the antimicrobial peptide LL37 acts as one of the brain’s defenses against microbes alongside beta-amyloid.
In order to test this hypothesis, the team injected the brains of mice that were bred to develop Alzheimer’s plaques comparable to the way humans do with bacteria. Overnight, the mice produced plaques. Tanzi discovered that each plaque had a single bacterium in it. A single bacterium can induce an entire plaque.
25 May 2016
Science Translational Medicine 25 May 2016:
Vol. 8, Issue 340, pp. 340ra72
DOI: 10.1126/scitranslmed.aaf1059
Amyloid-β peptide protects against microbial infection in mouse and worm models of Alzheimer’s disease
Phase 2
The Role of Sleep on the Brian’s Filtration System
Lulu Xie and team recently uncovered one of the key functions of sleep. The team compared 2 arousal states in the mouse, sleeping and awake, using state-of-the-art in vivo two-photon imaging. The researchers found that metabolic waste products of neural activity were cleared out of the sleeping brain at a faster rate than during the awake state. Like the accumulation of beta amyloid plaques, sleep is a cross-species phenomenon.
The study by Xie et. al reports that the critical function of metabolic homeostasis is dependant on sleep. The team utilized real-time assessments of tetramethylammonium diffusion and two-photon imaging in live mice. The experiment found a 60 percent increase in the interstitial space in association of natural sleep or anesthesia. This resulted in a noticeable convective exchange of cerebrospinal fluid and interstitial fluid, which are found in the spaces between the blood vessels and surrounding cells.
Thus as a result convective fluxes the rate of β-amyloid clearance during sleep. Hence, the restorative function of sleep may be a consequence of the enhanced removal of potentially neurotoxic waste products that accumulate during our waking hours.
Sleep Drives Metabolite Clearance from the Adult Brain
Science 18 Oct 2013:
Vol. 342, Issue 6156, pp. 373–377
DOI: 10.1126/science.1241224
In a separate study Ehsan Shokri-Kojori and team published a study on April 9 2018 showing that just 1 night of sleep deprivation builds β-Amyloid within the brain.
PNAS April 24, 2018 115 (17) 4483–4488

On September 5th of this year The Sleep Research Society came out with a report that collaborates with the data suggesting just how important the role of sleep is to prevent Alzheimer’s disease. The Baltimore Longitudinal Study of Aging, conducted by Adam P Spira et al showed that those who experienced excessive sleepiness during normal waking hours were nearly three times more likely to have brain deposits of beta amyloid than those who didn’t.
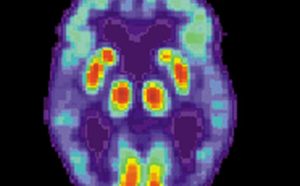
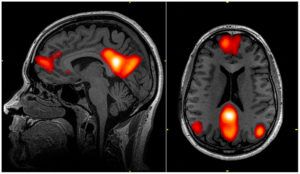
PET scan of a human brain with Alzheimer’s disease
Sleep, zsy152
Published: 05 September 2018
The accumulated evidence above shows that Beta-Amyloid are part of the brain’s ancient immune system . The evidence also indicates that the buildup of Beta-amyloid plaques occurs when it’s not flushed out. Many researchers, including Rudolf Tanzi and Robert Moir believe that it is the plaques that cause the damage to the brain.
Phase 3
The fIltration System of the Brain
The re-discovery of cellular membranes around the brain known as meningers has a network of lymphatic vessels that drain macromolecules from the CNS (cerebrospinal and interstitial fluids) into the cervical lymph nodes in mice. This lymphatic system was first discovered in 1787 (Mascagni, P. Vasorum lymphaticorum corporis humani historia et ichnographia) and was rediscovered by Da Mesquita et al.
Their research showed that meningeal lymphatics play a central role in brain health and disease by helping to maintaining both cognitive function and proteostasis.
The purpose of the lymphatic system in the body is to drain tissue of interstitial fluid that houses cellular debris and toxic chemicals. A rich protein-fluid called lymph is formed by interstitial fluids which circulates through the lymphatic system before returning to the blood. Lymph is filtered through the lymph nodes. Lymph nodes are responsible for initiating immune responses when foreign particles are detected.
Because the brain does not have its own lymphatic vessels it utilizes the interstitial fluid (ISF) along the walls of blood vessels to transport waste and proteins from the parenchyma to reach the cerebrospinal fluid that circulates through the meninges. The transvascular process is the process in which metabolic waste products, proteins, and other molecules in these fluids are removed from the brain by being transported along the walls of blood vessels, which crosses the blood-brain barrier. To what extent, if any, the meningeal lymphatic vessels were also involved in waste clearance is still unknown.
To verify the critical role of the meningeal lymphatic vessels Da Mesquite et. al injected a drug designed to damage the vessels into one of the three principal openings in the subarachnoid space between the arachnoid and pia mater layers of the meninges surrounding the brain, collectively referred to as the cisterna magna. In order to track the CSF they injected a fluorescent tracer molecule. This provided insight as to where the fluid goes in mice lacking meningeal lymphatic vessels. Da Mesquite et. al noted that the the tracer did not travel deep into the cervical lymph nodes in mice lacking meningeal lymphatic vessels. Injecting high concentrations of a tracer into the CSF can cause the diffusion of a tracer into the brain along blood vessels as shown by previous work — but this too was also reduced. The researchers used different tracers, surgically closing off drainage to the deep cervical lymph nodes; and examined mice with impaired lymphatic vessel development . The authors were able to confirm through these various methods the results by doing so.
The authors also noted that deficits within spatial orientation and memory were a result of meningeal lymphatic destruction.
Another interesting finding by the researchers was a change in gene expression within the hippocampus, a substructure crucial to memory and spatial orientation.
The gene expression resembled those previously observed in neurodegenerative disorders. The accumulated evidence by these experiments suggests collectively that the drainage of brain ISF and CSF by meningeal lymphatics is critical to proper function of cognition within the brain.
These findings present an interesting question: where exactly did the tracers go? One study (https://onlinelibrary.wiley.com/doi/abs/10.1038/icb.1951.30) suggests that tracers injected into the cisterna magna are transported primarily in the blood and that the lymphatic system is only secondary. To reveal whether the impairment of meningeal lymphatics can lead to a shift in the pathways responsible for controlling brain proteostasis the simultaneous measurements of tracers movements meningeal lymphatics, other lymphatic vessels, (for example the neck) and the blood may give insights into whether there’s an increase in the transvascular removal of waste products across the blood-brain barrier and/or their drainage into the venous system in the meninges.
The next observation by Da Mesquita’s team was a decrease in the diameter and coverage of meningeal lymphatic vessels through induced aging. The researchers also observed a decrease in drainage of tracers from the ISF and CSF into deep cervical lymph nodes. The signaling of the pathway involving vascular endothelial growth factor C (VEGF-C) and its receptor VEGFR3 promotes lymphatic vessel growth in mice. Likewise when the pathway involving endothelial growth was damaged it caused a loss of meningeal lymphatic vessels. Moreover the authors showed that there was an increase diameter of meningeal lymphatic vessels by providing treatment VEGF-C, improving lymphatic drainage as a result. Consistent with the accumulated research, Da Mesquita et al. showed that the drainage of CSF tracer could be restored into deep cervical lymph nodes by the local delivery of the vegf-c into the cisterna of old mice with a viral vector . This resulted in the restoration of spatial orientation.
The accumulation of amyloid-β protein within the brain, a hallmark of Alzheimer’s disease, causes age related impairments in the transvascular clearance of waste. The effects of of ablating the meningeal lymphatics in two mouse models of Alzheimer’s disease, in which amyloid-β protein is produced in neurons and secreted into the ISF was investigated by Da Mesquita and team. The results showed that ablation led to the accumulation of amyloid-β protein in the meningenges. The authors also noted to the potential relevance for humans by showing that amyloid-β had accumulated in meningenges of people who had Alzheimer’s disease as well.
When amyloid-β protein deposition in the brain parenchyma first became apparent, Da Mesquita and colleagues discovered that the two mouse models did not exhibit any apparent structural or functional changes in the meningeal lymphatics at the time. Cognitive impairment could not be prevented in either model using viral delivery vegf-c, which points to another disruption in another clearance pathway the early amyloid-β deposition and cognitive impairments in these two models-most likely the transvascular clearance. More than likely an increased burden is placed upon meningeal lymphatic system due to the gradual deterioration of transvascular-clearance routes through aging. Faulty lymphatic drainage of amyloid-β and other proteins from the ISF and CSF may be caused when the capacity of the lymphatic system is reached. This gives strong evidence to suggest that there is a very delicate relationship between meningeal lymphatics and blood vessels in the regulation of proteostasis in the brain.
In order to open new directions for research into cognition, neurodegeneration, and Alzheimer’s disease, the improvement of our understanding of waste clearance pathways from the brain, how the ISF and the CSF drain into the meningeal lymphatics, and how these lymphatic vessels interact with the blood vessels at the blood-brain barrier is vital. The potential of improving clearance with meningeal lymphatics to rebuild brain proteostasis, and may lessen amyloid-β protein deposition have been shown by Da Mesquita et al. to rely upon the local growth of lymphatic vessels. Whether or not the improvement of the impaired function of blood vessels due to aging, and whether enhancing clearance at the blood-brain barrier can improve lymphatic drainage function, remains to be to determined.
Nature 25 July 2018
A lymphatic-waste disposal system implicated in Alzheimer’s Disease
Naturevolume 560, pages185–191 (2018
Functional aspects of meningeal lymphatics in ageing and Alzheimer’s disease
The accumulated evidence shows that Beta-amyloid may prove to be beneficial in killing off microbes, but also that proper rest is needed to activate the filtration system of the brain to consistently flush out these neurotoxins before they cause impairment or disease . Further research into the prevention of age-related damage to the brain’s lymphatic system may offer clues as to how we can prevent cognitive decline of age-related diseases. Ideally this will be done with protective mechanisms the brain already has in place.
Journal of Alzheimer’s Disease — Volume Pre-press, issue Pre-press, vol. 65, no. 2, pp. 659–682, 2018
Accepted 5 July 2018
Published: 21 August 2018
Postsynaptic Proteome of Non-Demented Individuals with Alzheimer’s Disease Neuropathology
Further analysis of these key factors could pave the road to ending Alzheimer’s and other age-related diseases.
Nicholi Avery, [email protected]
Adam Alonzi, [email protected]

Investigators at Harvard Medical School have identified the key cellular mechanisms behind vascular aging and its effects on muscle health, and they have successfully reversed the process in animals.
The scientists used a chemical compound that’s an NAD+ booster called NMN which plays a critical role in repairing cellular DNA as well as maintaining cell vitality to test what would happen.
Could reversing the aging of blood vessels hold the key to restoring youthful vitality? If the old adage “you are as old as your arteries” reigns true then the answer is yes, at least in mice.
According to a new study by Harvard Medical School researchers, they have identified the cellular mechanisms that cause the aging of vascular arteries as well as the effects of such aging on the health of muscles. The Medical team was also able to successfully reverse this aging process.
What these findings seem to indicate is that there’s a glitch in the normal crosswalk between both muscles and blood vessels and keeping both tissues healthy. The scientists were also able reverse the demise of blood vessels and muscle atrophy in the aging mice by using the synthetic precursors of two molecules naturally present in the body. This boosted their exercise endurance in the process.
The Medical team is excited because such a breakthrough will now pave the way to identifying new therapies for humans.
Study senior investigator David Sinclair, professor in the Department of Genetics at Harvard Medical School and co-director of the Paul F. Glenn Center for the Biology of Aging at Harvard Medical School stated “we’ve discovered a way to reverse vascular aging by boosting the presence of naturally occurring molecules in the body that augment the physiological response to exercise.”
Because there are some very important differences in biology between humans and mice there’s a possibility that this treatment may not have the same effect in humans. Nonetheless, the research team plans to follow through with human clinical trials because the results of this experiment were important enough to prompt the research team in doing so.
Sinclair, who is also a professor at the University of New South Wales School of Medical Sciences in Sydney, Australia stated, “the approach stimulates blood vessel growth and boosts stamina and endurance in mice and sets the stage for therapies in humans to address the spectrum of diseases that arise from vascular aging.”
One of the side effects of aging is reduced blood flow and the compromise of oxygenation of organs and tissue because our tiniest blood vessels began to wither and die. Cardiac and neurologic conditions, muscle loss, impaired wound healing and overall frailty, and among other things are the results of vascular aging. As these blood vessels die there’s a loss of blood flow to organs and tissues which causes toxins build-up and a loss of oxygen.
For quite some time scientists have known the essential role that endothelial cells, which line blood vessels, play in the health and growth of blood vessels that supply oxygen-rich and nutrient-loaded blood to organs and tissues. Unfortunately, as with all things on the human body, these endothelial cells age having a detrimental effect on the body. New blood vessels fail to form, blood vessels atrophy, and the overall blood flow to most parts of the body diminishes. This has a powerful impact on muscles, which heavily rely on robust blood supply to function because they’re heavily vascularized.
Typically we exercise in hopes of slowing down sarcopenia, but unfortunately even that doesn’t last forever. Gradually our muscles grow weaker and begin to shrivel as part of the aging process.
What precisely curtails the blood flow and precipitates this unavoidable decline? Why does even exercise lose its protective power to sustain muscle vitality? Is this process reversible? There were some of the leading questions Sinclair and team had.
The Experiment/Results:
Sinclair and his team discovered through a series of experiments that the flow of blood is reduced as endothelial cells start to lose a critical protein known as sirtuin1, or SIRT1. SIRT1 delays aging and extends life in yeast and mice as shown in previous studies.
Research done previously by Sinclair and others has shown that NAD+ boosts the activity of SIRT1. SIRT1 loss is a result of the loss of NAD+, which is a key regulator of protein interactions and DNA repair that was identified more than a century ago. As NAD+ declines with age so does the protein SIRT1.
The results showed that the critical interface that enables the conversation between endothelial cells in the walls of blood vessels and muscle cells is provided by NAD+ and SIRT1.
SIRT1 signaling is activated and generates new capillaries, the tiniest blood vessels in the body that supply oxygen and nutrients to tissues and organs in young mice muscles. As the mice aged, however, the study found that muscle tissue was left nutrient-deprived and oxygen-starved as a result in the diminishment of NAD+ and SIRT1.
The researchers hope that their findings may pave the way to therapeutic advances that hold promise for the millions of older people for whom regular physical activity is not an option.
Abhirup Das, the studies first author, who conducted the work as a post-doctoral fellow in Sinclair’s lab, currently a visiting scholar in genetics at Harvard Medical School and a post-doctoral research fellow at the University of South New Wales School of Medical Sciences, said that “we reasoned that declining NAD+ levels reduce SIRT1 activity and thus interfere with aging mice’s ability to grow new blood vessels.”
The researchers then set their sights on the NAD+, which is a critical coenzyme for enzymes that fuel reduction-oxidation reactions, carrying electrons from one reaction to another, and as a cosubstrate for other enzymes such as the sirtuins and poly(adenosine diphosphate-ribose) polymerases.
The scientists used a chemical compound that’s an NAD+ booster called NMN (no not m&m!) which plays a critical role in repairing cellular DNA as well as maintaining cell vitality to test what would happen.
One of the results showed that treatment from NMN caused endothelial cells from humans and mice to have strong growth capacity and reduced cell death.
The team then wondered what would happen to a group of mice that were 20 months old—the rough equivalent of 70 in human years given NMN. After a 2 month time span the results showed that NMN treatment restored the number of blood capillaries and capillary density to those seen in younger mice. Blood flow to the muscles also increased and was significantly higher than blood supply to the muscles seen in same-age mice that didn’t receive NMN.
That wasn’t the most surprising result to the researchers however. What they discovered was that the aging mice showed in comparison to the entreated mice that they regained the capacity to exercise by 56 and 80% more. The untreated mice could only run 240 meters, or 780 feet, on average whereas the mice treated with NMN could run 430 meters, or about 1,400 feet, on average. This treatment could be an answer to humans who have lost the capacity to exercise due to other disabilities or age-related diseases.
The next step for the researchers was to explore methods for boosting the activity of SIRT1. To do this the researchers added a second compound NaHS, sodium hydrosulfide, which is known to be a precursor of SIRT1.
For four weeks a group of mice that were 32-month-old mice—the rough equivalent to 90 in human years—receiving the combo treatment. The results were significant! Not only were the mice able to run longer and faster but they were able to outperform the untreated mice by a longshot. The treated mice ran 1.6 times further than the untreated mice.
Study co-author James Mitchell, associate professor of genetics and complex diseases at the Harvard T. H. Chan School of Public Health stated that “these are really old mice so our finding that the combo treatment doubles their running capacity is nothing short of intriguing.”
“This observation underscores the notion that age plays a critical role in the crosstalk between blood vessels and muscles and points to a loss of NAD+ and SIRT1 as the reason behind loss of exercise effectiveness after middle age,” Das said.
One of the ultimate goals for the team is to eventually move forward in developing small-molecule, NMN-based drugs that mimic the effects of exercise—enhanced blood flow and oxygenation of muscles and other tissues. Though they must first replicate their findings first. Such therapies could potentially help with new vessel growth of organs that suffer tissue-damaging loss of blood supply and oxygen, a common scenario in heart attacks and ischemic strokes, the team said.
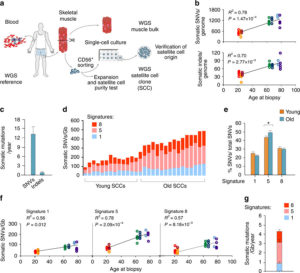
Study based upon human skeletal muscle aging, mutagenesis, and the role of #satellite cells.
“A more comprehensive understanding of the interplay of stem cell–intrinsic and extrinsic factors will set the stage for improving cell therapies capable of restoring tissue homeostasis and enhancing muscle repair in the aged.”
Human aging has multiple effects on the human body. One of the effects of human aging is the reduction in skeletal muscle (SkM) function and a reduction in the number and activity of satellite cells (SCs), the resident stem cells. The whole genome of single SC clones of the leg muscle vastus lateralis from healthy individuals of different ages (21–78 years) was analyzed, to study the specific connection between SC aging and muscle impairment. In healthy adult muscle rapid increase of SCs is consistent with the accumulation rate of 13 somatic mutations per genome per year. Mutations typically do not happen in SkM-expressed genes because they are protected. However, as mutations in exons and promoters increase, genes involved in SC activity and muscle function are targeted which results in aging. Exons are coding sections of an RNA transcript, or the DNA encoding it, that are translated into protein. Proteins are the synthesis of molecules. A change in of a single base pair that caused the substitution of a different amino acid in the resulting protein (missense mutation) that was propagated to the muscle and detected in association with SC mutations affecting the whole tissue. #Somatic mutagenesis in SCs as a result is the driving force in the age related decline of SkM function.
Satellite Cells
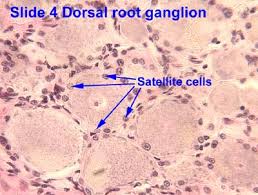
Satellite cells (SCs) are a heterogeneous population of stem and progenitor cells. These cells play an important role in the growth and development of myofiber. The enlargement, regeneration, and remodeling in skeletal muscle (SkM) is the pivotal role of satellite cells. Satellite cells are dormant until they become activated through exercise or SkM injury. Upon injury skeletal muscle have a remarkable ability to recover from injury. Skeletal muscle goes through a sophisticated degeneration and regenerative process that takes place at the tissue, cellular, and molecular levels. This regenerative process relies upon the dynamic interplay between satellite cells and their environment (stem cell niche). SCs multiply further when committed to myogenic differentiation. As SCs proliferate further they begin to combine with existing SkM fibers and supply new nuclei to the growing and regenerating fibers. The declining of numbers of proliferative potential of SCs is one sign of aging in human SkMs.
A flawed SC compartment is foreseen as a major contributor for age-related deficiencies such as, skeletal muscle tissue having restricted mobility and voluntary functions. The results of such defects include a reduced capacity to respond to hypertrophic stimuli such as exercise and impaired recovery from muscle disuse and injury and the disruption of muscle tissue homeostasis. Moreover, the SCs of nonactive adult animals have been shown to contribute to differentiated fibers in non-injured muscles. Less important is the basal turnover of nuclei in adult fibers in the protection from sarcopenia. This hypothesis was tested and showed that lifelong reduction of satellite cells neither accelerated nor exacerbated sarcopenia and that satellite cells did not contribute to the maintenance of muscle size or fiber type composition during aging, but that their loss may contribute to age-related muscle fibrosis. The progressive loss of SkM mass and function known as sarcopenia affects up to 29% of the population aged 85 years. The accumulation of sarcopenia causes a highly disabling condition. It is essential, nonetheless, that the characterization of SCs in human pathology be further explored. SCs are a key factor in limiting the occurrence of fibrosis in the SkM of mice affected by sarcopenia.
The progressive loss of SkM mass and function known as sarcopenia affects up to 29% of the population aged 85 years. The accumulation of sarcopenia causes a highly disabling condition. It is essential, nonetheless, that the characterization of SCs in human pathology be further explored. Scs are key in limiting the occurrence of fibrosis in the SkM of mice affected by sarcopenia. Genome integrity is essential for the function of stem-cells. But there still must be some stability of the genome. Genetic mutations in the soma has diverse physiological roles and pathological consequences, such as the decline of stem-cell functions. Starting from the first division of the embryo, modifications in the genome extend from single-base changes (single-nucleotide variants (SNVs)) to insertions or deletions of a few bases (indels) to chromosomal rearrangements and occur during the whole life. Somatic variants are not propagated to the whole individual but to a subpopulation of cells in the body, which is strikingly different from germline variants. Adult human tissues become a mosaic of genetically different cells as a result. Furthermore, as a result of the buildup of errors taking place either during cell-division or because of environmental induced DNA damage, somatic mutation burden increases, causing age-related disease. Currently, somatic mutation burden in human SCs or SkM is unknown.
The purpose of the investigation of genetic alterations that occur with aging in the genome of human adult SCs is to use the results to clearly explain mutational processes and SC replication rate occurring in vivo in adult human muscles. The prediction of global consequences on muscle aging and sarcopenia was done by evaluating the functional effects of somatic mutations on SC proliferation and differentiation.
Results
Resources:
“Somatic mutagenesis in satellite cells associates with human skeletal muscle aging.”
Nature Communications volume 9, Article number: 800(2018) Full Abstract Study
“Satellite Cells and the Muscle Stem Cell Niche.”
Physiological Reviews Volume 93, No.1 (2013) Physiological Reviews
“Tissue-specific mutation accumulation in human adult stem cells during life.”
Nature International Journal of Science volume 538, pages 260–264 (13 October 2016) Abstract Study
“When stem cells grow old: phenotypes and mechanisms of stem cell aging”
Development for advances in developmental biology and stem cells Development 2016 143: 314 Abstract Study “Clock-like mutational processes in human somatic cells.”
Nature Genetics volume 47, pages 1402–1407 (2015) Abstract Study