“If we intervene early, the treatments can kick in early and slow down the progression of the disease and at the same time avoid more damage,” Prof Zoe Kourtzi of Cambridge University said.



Circa 2018
Mechanical loading is an important cue for directing stem cell fate and engineered tissue formation in vitro. Stem cells cultured on 2-dimensional (D) substrates and in 3D scaffolds have been shown to differentiate toward bone, tendon, cartilage, ligament, and skeletal muscle lineages depending on their exposure to mechanical stimuli. To apply this mechanical stimulus in vitro, mechanical bioreactors are needed. However, current bioreactor systems are challenged by their high cost, limited ability for customization, and lack of force measurement capabilities. We demonstrate the use of 3-dimensional printing (3DP) technology to design and fabricate a low-cost custom bioreactor system that can be used to apply controlled mechanical stimuli to cells in culture and measure the mechanical properties of small soft tissues. The results of our in vitro studies and mechanical evaluations show that 3DP technology is feasible as a platform for developing a low-cost, customizable, and multifunctional mechanical bioreactor system.
• 3DP technology was used to print a multifunctional bioreactor system/tensile load frame for a fraction of the cost of commercial systems.
• The system mechanically stimulated cells in culture and evaluated the mechanical properties of soft tissues.

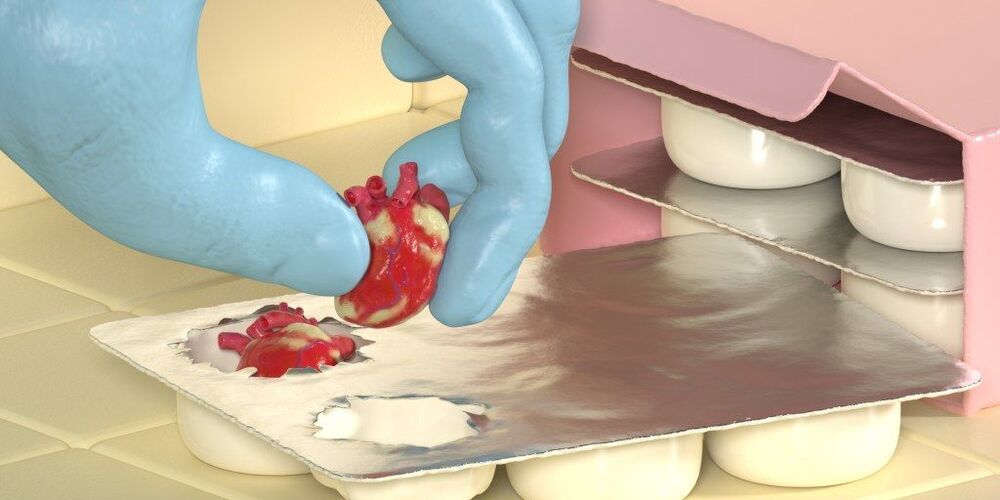
Scientists at the Catholic University of Korea and Asan Medical Center have 3D printed a novel device that could be used to stabilize Acute Liver Failure (ALF) patients as they wait for a life-saving organ donation.
Composed of a 3D printed container and semipermeable membrane, the team’s implant allows for the rapid delivery of the drugs needed to save those in danger of succumbing to ALF. Once implanted, the device also acts as a ‘bioartificial support system,’ functioning as the patient’s liver while reducing any dysfunction caused to their other internal organs, keeping them alive until a transplant becomes available.
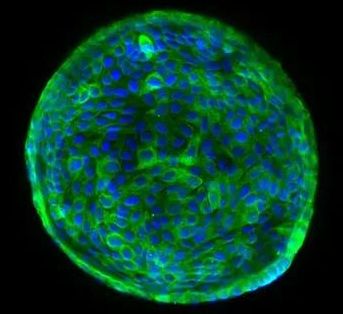
Researchers determined that when introduced into damaged mouse or donated human livers, these lab-grown tissues could integrate into bile ducts and function normally.
ABOVE: A human cholangiocyte–derived organoid with nuclei in blue and the cytoplasm of bile duct cells in green FOTIOS SAMPAZIOTIS, TERESA BREVINI
Scientists have shown over the past decade or so that organoids—small, organ-like structures grown in culture from stem cells—can integrate into many organs, including the liver, lungs, and guts of mice, and repair defects. In a study published today (February 18) in Science, researchers have advanced this approach in human tissue, and demonstrate that organoids derived from adult cholangiocytes, the cells that line the bile ducts, can integrate into human livers from deceased organ donors. The findings pave the way for new treatments for liver diseases, as well as for the repair of donated organs to make more available for transplant.
“It is quite spectacular if you can really functionally repair the liver by injecting cholangiocytes into an intact liver,” says Hans Clevers, a developmental biologist at Utrecht University in the Netherlands. He was not involved in the work, but in research led by former postdoc Meritxell Huch, his group showed in 2015 that it was possible to grow human liver organoids in culture and that they could be successfully transplanted into mice—work the authors of the new study have built upon.

The ones Teresa is handling in this Cambridge laboratory are mini bile ducts, thin tubes that carry bile from the liver to the small intestine to help with digestion.
Teresa also has gut organoids in the incubator, while down the corridor a different team is developing brain organoids.
In fact, around the world, miniatures of everything from lungs to kidneys are being coaxed gently to life. And because they function just as organs do, they are perfect for research.
By examining MRI data from a large Open Science repository, researchers reconstructed a brain connectivity pattern, and applied it to an artificial neural network (ANN). An ANN is a computing system consisting of multiple input and output units, much like the biological brain.
Artificial neural networks modeled on human brain connectivity can effectively perform complex cognitive tasks.