To keep the longevity train rolling it may not be enough to cure diseases. We may also need to address the underlying condition of aging itself, which is, after all, the primary risk factor for late-life decline.
What happens if we slow down aging?


Enrolling Tens Of Thousands Of Dogs, In 10-Year Study, To Unlock Healthy Aging Secrets — Dr Matt Kaeberlein, Founder / Co-Director, The Dog Aging Project, Professor, University of Washington, joins me on Progress, Potential, And Possibilities Nathan Shock Centers #Rapamycin #Dogs #Aging #Longevity #Healthspan #Geroscience.
#MitochondrialDisease
Dr. Matt Kaeberlein is Professor of Pathology, Adjunct Professor of Genome Sciences, and Adjunct Professor of Oral Health Sciences, at the University of Washington.
Dr. Kaeberlein received his PhD from MIT in Biology, did his post-doc in the Department of Genome Sciences, University of Washington, and his research interests are focused on basic mechanisms of aging in order to facilitate translational interventions that promote healthspan and improve quality of life.
Dr. Kaeberlein has published nearly 200 papers in top scientific journals and has been recognized by several prestigious awards, including a Breakthroughs in Gerontology Award, an Alzheimer’s Association Young Investigator Award, an Ellison Medical Foundation New Scholar in Aging Award, a Murdock Trust Award, a Pioneer in Aging Award, and the Vincent Cristofalo Rising Star in Aging Research.
Dr. Kaeberlein’s contributions have also been recognized with Fellow status in the American Association for the Advancement of Science, the American Aging Association, and the Gerontological Society of America.
Dr. Kaeberlein is a past President of the American Aging Association and has served on their Executive Committee and Board of Directors since 2012. He has also served as a member of the Board of Directors for the Federation of American Societies for Experimental Biology and is currently the Chair of the Biological Sciences Section of the Gerontological Society of America.
Dr. Kaeberlein serves on the editorial boards for several journals, including Science and eLife. Dr. Kaeberlein’s scientific discoveries have generated substantial public interest, with featured stories in major media outlets including appearing on the front page of the New York Times, the Today Show, CNN, the UK Telegraph, Popular Science, Time Magazine, Scientific American, NPR, USA Today, National Geographic, and many others.
In addition to his primary appointments, Dr. Kaeberlein is the co-Director of the University of Washington Nathan Shock Center of Excellence in the Basic Biology of Aging, the Founding Director of the Healthy Aging and Longevity Research Institute at the University of Washington, and Founder and Co-Director of the Dog Aging Project.
The latest from Calico. A bit technical.
Reprogramming of ordinary somatic cells into induced pluripotent stem cells (iPSCs) was initially thought to be a way to obtain all of the patient matched cells needed for tissue engineering or cell therapies. A great deal of work has gone towards realizing that goal over the past fifteen years or so; the research community isn’t there yet, but meaningful progress has taken place. Of late, another line of work has emerged, in that it might be possible to use partial reprogramming as a basis for therapy, delivering reprogramming factors into animals and humans in order to improve tissue function, without turning large numbers of somatic cells into iPSCs and thus risking cancer or loss of tissue structure and function.
Reprogramming triggers some of the same mechanisms of rejuvenation that operate in the developing embryo, removing epigenetic marks characteristic of aged tissues, and restoring youthful mitochondrial function. It cannot do much for forms of damage such as mutations to nuclear DNA or buildup of resilient metabolic waste, but the present feeling is there is nonetheless enough of a potential benefit to make it worth developing this approach to treatments for aging. Some groups have shown that partial reprogramming — via transient expression of reprogramming factors — can reverse functional losses in cells from aged tissues without making those cells lose their differentiated type. But this is a complicated business. Tissues are made up of many cell types, all of which can need subtly different approaches to safe reprogramming.
Today’s open access preprint is illustrative of the amount of work that lies ahead when it comes to the exploration of in vivo reprogramming. Different cell types behave quite differently, will require different recipes and approaches to reprogramming, different times of exposure, and so forth. It makes it very hard to envisage a near term therapy that operates much like present day gene therapies, meaning one vector and one cargo, as most tissues are comprised of many different cell types all mixed in together. On the other hand, the evidence to date, including that in the paper here, suggests that there are ways to create the desired rejuvenation of epigenetic patterns and mitochondrial function without the risk of somatic cells dedifferentiating into stem cells.


Aging is associated with an overall decline in health and increased frailty, and is a major risk factor for multiple chronic diseases. Frailty syndrome, characterized by weakness, fatigue and low physical activity, affects more than 30% of the elderly population. Increasing our understanding of the mechanisms underlying the aging process is a top priority to facilitate the development of interventions that will lead to the preservation of health and improvements on survival and lifespan.
Cumulative evidence suggests that diet and metabolism are key targetable regulators of healthy lifespan. Prof. Haim Cohen, Director of the Sagol Healthy Human Longevity Center at Bar-Ilan University, focuses much of his research on the SIRT6 protein that is involved in regulating many biological processes, such as aging, obesity, and insulin resistance.
In a study just published in the journal Nature Communications, an international team led by Cohen and his Ph.D. student Asael Roichman—together with Prof. Rafael de Cabo, of the National Institute on Aging at the National Institutes of Health, Prof. Manuel Serrani, of the Institute for Research in Biomedicine in Barcelona, and Prof. Eyal Gottlieb from the Technion—report that transgenic mice express high levels of the SIRT6 gene, and show that their life expectancy can be increased by an average of 30% in both males and females. Translated into human terms this means that a 90-year-old could live until nearly 120!
A new discovery could lead to new drugs for faster repairing muscles after injury — or rebuilding muscle mass lost during the normal aging process.
Researchers at the Salk Institute have uncovered a mechanism by which stem cells can help regenerate muscles. The discovery could provide a new drug target for repairing muscles after injury or rebuilding muscle mass lost during the normal aging process.
The breakthrough started with a set of proteins called Yamanaka factors, which have long been studied as a key part of stem cell therapy. These factors are used to convert regular cells – most commonly skin cells – into what are known as induced pluripotent stem cells (iPS), which can then go on to differentiate into a variety of other cell types. That in turn helps regenerate tissue. But exactly how the Yamanaka factors worked their magic remained a mystery.
“Our laboratory previously showed that these factors can rejuvenate cells and promote tissue regeneration in live animals,” says Chao Wang, first author of the study. “But how this happens was not previously known.”
Hydraulic Instability Decides Who’s to Die and Who’s to Live
In many species including humans, the cells responsible for reproduction, the germ cells, are often highly interconnected and share their cytoplasm. In the hermaphrodite nematode Caenorhabditis elegans, up to 500 germ cells are connected to each other in the gonad, the tissue that produces eggs and sperm. These cells are arranged around a central cytoplasmic “corridor” and exchange cytoplasmic material fostering cell growth, and ultimately produce oocytes ready to be fertilized.
In past studies, researchers have found that C. elegans gonads generate more germ cells than needed and that only half of them grow to become oocytes, while the rest shrinks and die by physiological apoptosis, a programmed cell death that occurs in multicellular organisms. Now, scientists from the Biotechnology Center of the TU Dresden (BIOTEC), the Max Planck Institute of Molecular Cell Biology and Genetics (MPI-CBG), the Cluster of Excellence Physics of Life (PoL) at the TU Dresden, the Max Planck Institute for the Physics of Complex Systems (MPI-PKS), the Flatiron Institute, NY, and the University of California, Berkeley, found evidence to answer the question of what triggers this cell fate decision between life and death in the germline.

With 2700 locations across 10000 U.S. communities, YMCA is becoming a major hub for healthy living — From vaccinations and diabetes prevention programs, to healthy aging and wellness — Siva Balu, VP/Chief Information Officer — The Y of the U.S.A.
Mr. Siva Balu is Vice President and Chief Information Officer of YMCA of the U.S. (Y-USA), where he is working to rethink and reorganize the work of the organization’s information technology strategy to meet the changing needs of Y-USA and Ys throughout the country.
The YMCA is a leading nonprofit committed to strengthening community by connecting all people to their potential, purpose and each other, with a focus on empowering young people, improving health and well-being and inspiring action in and across communities, and with presence in 10000 neighborhoods across the nation, they have real ability to deliver positive change.
Mr. Balu has 20 years of healthcare technology experience in leadership roles for Blue Cross Blue Shield, the nation’s largest health insurer, which provides healthcare to over 107 million members—1 in 3 Americans. He most recently led the Enterprise Information Technology team at the Blue Cross Blue Shield Association (BCBSA), a national federation of Blue Cross and Blue Shield companies.
Mr. Balu was responsible for leading all aspects of IT, including architecture, application and product development, big data, business intelligence and data analytics, information security, project management, digital, infrastructure and operations. He has created several highly scalable innovative solutions that cater to the needs of members and patients throughout the country in all communities. He provided leadership in creating innovative solutions and adopting new technologies for national and international users.
Mr. Balu earned a bachelor’s degree in electronics and communication engineering from Bharathiar University in India, a master’s in business administration from Lake Forest Graduate School of Management and executive master’s degree from MIT-Sloan School of Management in Innovation, Strategy and Artificial Intelligence as well as additional certifications in Disruptive Strategy and Negotiation Mastery from Harvard Business School Online.
In his free time, Mr. Balu volunteers and contributes to several charities, including The Soondra Foundation (delivering healthcare to the working poor in India), Special Olympics, Chicago Food Depository, Sarah’s Inn, Challenged Athletes Foundation, Beyond Hunger, The Pack Shack, Cradles to Crayons and Gardeneers.
Mr. Balu has also developed a passion for long-distance running a few years ago starting with a 5k, and then to marathons and to running multiple ultra-marathons. He has run multiple 100-mile races. He recently ran what is referred to as ‘the worlds toughest foot race,’ Badwater 135-miler in Death Valley, and one of the coldest races, Tuscobia 160-miler.
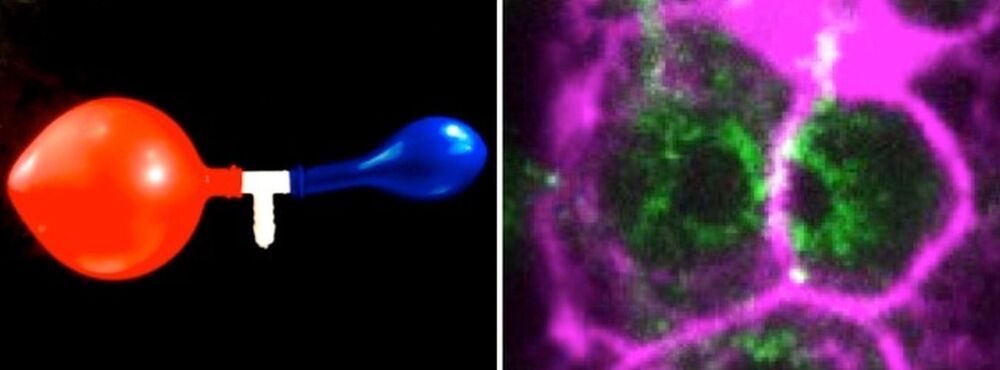
In many species including humans, the cells responsible for reproduction, the germ cells, are often highly interconnected and share their cytoplasm. In the hermaphrodite nematode Caenorhabditis elegans, up to 500 germ cells are connected to each other in the gonad, the tissue that produces eggs and sperm. These cells are arranged around a central cytoplasmic “corridor” and exchange cytoplasmic material fostering cell growth, and ultimately produce oocytes ready to be fertilized.
In past studies, researchers have found that C. elegans gonads generate more germ cells than needed and that only half of them grow to become oocytes, while the rest shrink and die by physiological apoptosis, a programmed cell death that occurs in multicellular organisms. Now, scientists from the Biotechnology Center of the TU Dresden (BIOTEC), the Max Planck Institute of molecular Cell Biology and Genetics (MPI-CBG), the Cluster of Excellence Physics of Life (PoL) at the TU Dresden, the Max Planck Institute for the Physics of Complex Systems (MPI-PKS), the Flatiron Institute, NY, and the University of California, Berkeley, have found evidence to answer the question of what triggers this cell fate decision between life and death in the germline.
Prior studies revealed the genetic basis and biochemical signals that drive physiological cell death, but the mechanisms that select and initiate apoptosis in individual germ cells remained unclear. As germ cells mature along the gonad of the nematode, they first collectively grow in size and in volume homogenously. In the study just published in Nature Physics, the scientists show that this homogenous growth suddenly shifts to a heterogenous growth where some cells become bigger and some cells become smaller.

TIL a preprint publication points to another commonality found in blue zones: their lack of birth records. Author Dr. Saul Justin Newman concludes, “the designated ‘blue zones’ of Sardinia, Okinawa, and Icaria corresponded to regions with low incomes, low literacy, high crime rate and short life expectancy relative to their national average. As such, relative poverty and short lifespan constitute unexpected predictors of centenarian and supercentenarian status, and support a primary role of fraud and error in generating remarkable human age records.”
Can the blue zone diet help with longevity? We investigate Dan Buettner’s claims about blue zones and the corresponding lifestyle.