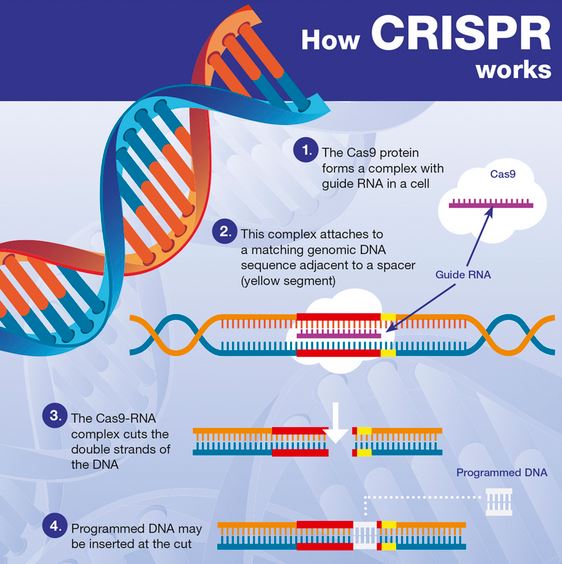
Current optical techniques can image neuron activity only near the brain’s surface, but integrated neurophotonics could unlock circuits buried deep in the brain. Credit: Roukes et. al.
But current optogenetic studies of the brain are constrained by a significant physical limitation, says Laurent Moreaux, Caltech senior research scientist and lead author on the paper. Brain tissue scatters light, which means that light shone in from outside the brain can travel only short distances within it. Because of this, only regions less than about two millimeters from the brain’s surface can be examined optically. This is why the best-studied brain circuits are usually simple ones that relay sensory information, such as the sensory cortex in a mouse—they are located near the surface. In short, at present, optogenetics methods cannot readily offer insight into circuits located deeper in the brain, including those involved in higher-order cognitive or learning processes.
Integrated neurophotonics, Roukes and colleagues say, circumvents the problem. In the technique, the microscale elements of a complete imaging system are implanted near complex neural circuits located deep within the brain, in regions such as the hippocampus (which is involved in memory formation), striatum (which controls cognition), and other fundamental structures in unprecedented resolution. Consider the similar technology of functional magnetic resonance imaging (fMRI), the scanning technique currently used to image entire brains. Each voxel, or three-dimension pixel, in an fMRI scan is typically about a cubic millimeter in volume and contains roughly 100,000 neurons. Each voxel, therefore, represents the average activity of all of these 100,000 cells.