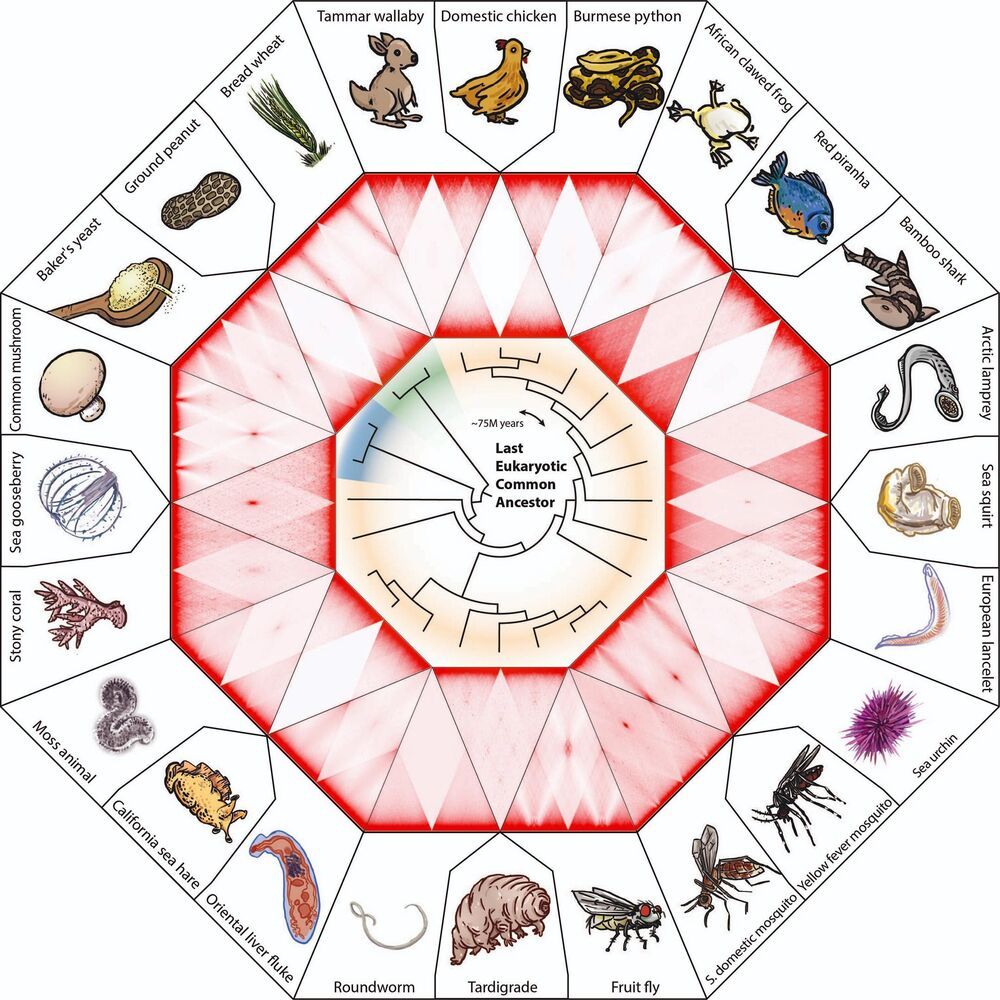
One hundred fifty years ago, Dmitri Mendeleev created the periodic table, a system for classifying atoms based on the properties of their nuclei. This week, a team of biologists studying the tree of life has unveiled a new classification system for cell nuclei, and discovered a method for transmuting one type of cell nucleus into another.
The study, which appears this week in the journal Science, emerged from several once-separate efforts. One centered on the DNA Zoo, an international consortium spanning dozens of institutions including Baylor College of Medicine, the National Science Foundation-supported Center for Theoretical Biological Physics (CTBP) at Rice University, the University of Western Australia and SeaWorld.
Scientists on the DNA Zoo team had been working together to classify how chromosomes — which can be several meters long — fold up to fit inside the nuclei of different species from across the tree of life.