Circa 2017
Learn how Stanford Health Care brings together leading-edge technology, innovative research, and world-renowned experts to meet your unique needs.


Aging is associated with an overall decline in health and increased frailty, and is a major risk factor for multiple chronic diseases. Frailty syndrome, characterized by weakness, fatigue and low physical activity, affects more than 30% of the elderly population. Increasing our understanding of the mechanisms underlying the aging process is a top priority to facilitate the development of interventions that will lead to the preservation of health and improvements on survival and lifespan.
Cumulative evidence suggests that diet and metabolism are key targetable regulators of healthy lifespan. Prof. Haim Cohen, Director of the Sagol Healthy Human Longevity Center at Bar-Ilan University, focuses much of his research on the SIRT6 protein that is involved in regulating many biological processes, such as aging, obesity, and insulin resistance.
In a study just published in the journal Nature Communications, an international team led by Cohen and his Ph.D. student Asael Roichman—together with Prof. Rafael de Cabo, of the National Institute on Aging at the National Institutes of Health, Prof. Manuel Serrani, of the Institute for Research in Biomedicine in Barcelona, and Prof. Eyal Gottlieb from the Technion—report that transgenic mice express high levels of the SIRT6 gene, and show that their life expectancy can be increased by an average of 30% in both males and females. Translated into human terms this means that a 90-year-old could live until nearly 120!
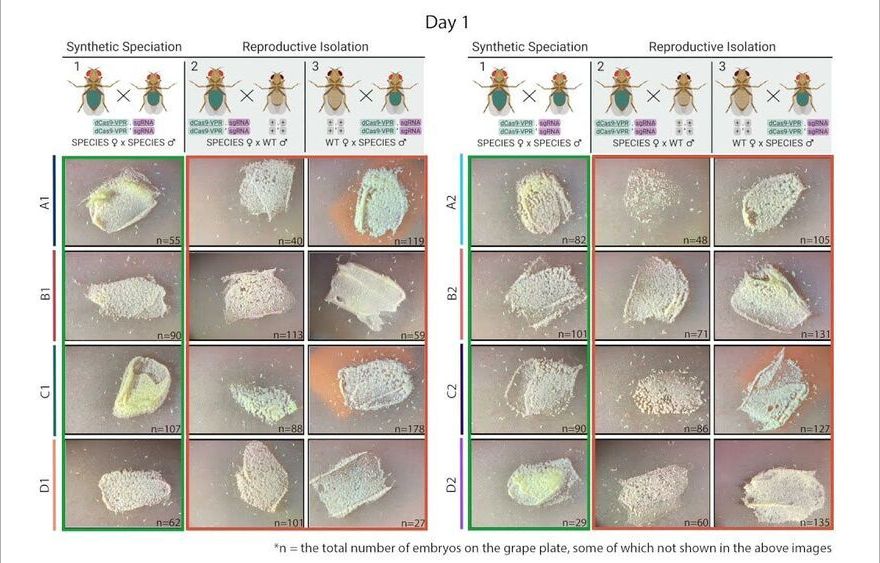
CRISPR-based technologies offer enormous potential to benefit human health and safety, from disease eradication to fortified food supplies. As one example, CRISPR-based gene drives, which are engineered to spread specific traits through targeted populations, are being developed to stop the transmission of devastating diseases such as malaria and dengue fever.
But many scientists and ethicists have raised concerns over the unchecked spread of gene drives. Once deployed in the wild, how can scientists prevent gene drives from uncontrollably spreading across populations like wildfire?
Now, scientists at the University of California San Diego and their colleagues have developed a gene drive with a built-in genetic barrier that is designed to keep the drive under control. Led by molecular geneticist Omar Akbari’s lab, the researchers engineered synthetic fly species that, upon release in sufficient numbers, act as gene drives that can spread locally and be reversed if desired.
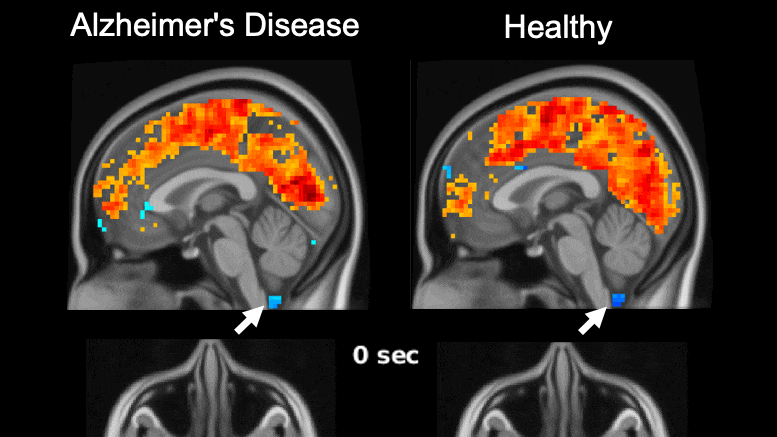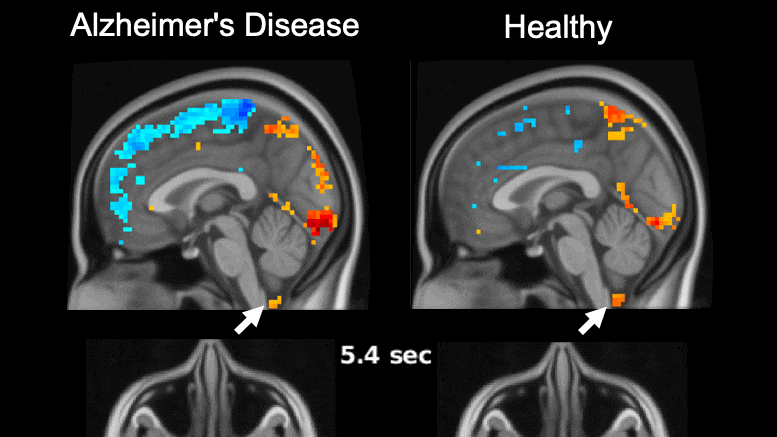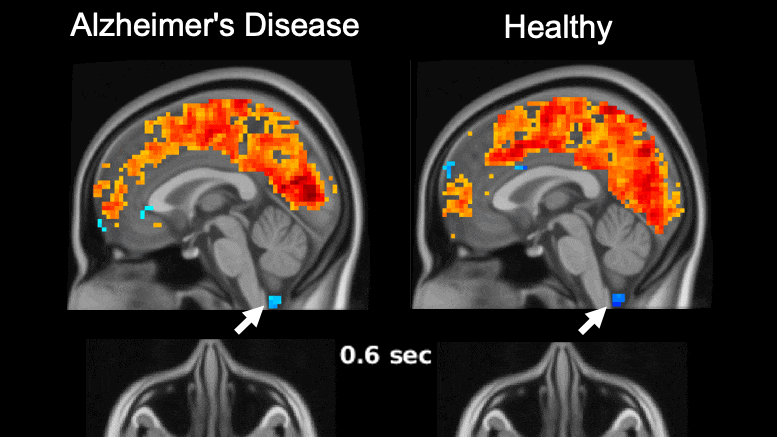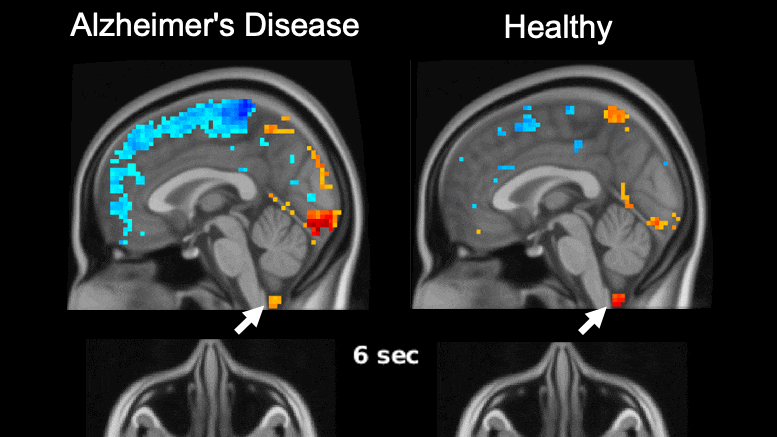
Global brain activity seen on fMRI, and its connection with cerebrospinal fluid flow weaker in brains of individuals with Alzheimer’s disease risk or related toxin buildup.
Evidence of sleep-dependent low-frequency (0.1 Hz) global brain activity in the clearance of Alzheimer’s disease-related toxin buildup is presented in research published today (June 1, 2021) in the open access journal PLOS Biology by Xiao Liu and colleagues at The Pennsylvania State University. This neuronal activity was more strongly linked with cerebrospinal fluid flow in healthy controls than higher risk groups and patients, and the findings could serve as a potential imaging marker for clinicians in evaluating patients.
The development of Alzheimer’s disease is believed to be driven by the buildup of the toxic proteins amyloid-β and tau in the brain. The brain’s glymphatic system plays a crucial role in clearing these toxins and previous work has shown a possible relationship between sleep-dependent global brain activity and the glymphatic system by showing this activity is coupled by cerebrospinal fluid flow essential for the glymphatic system.

I believe I posted about Nitric Oxide as treatment for covid19 ages ago. Apparently I was right. It also works under the UK variant, thus showing it can work under others as well.
Results of clinical trials conducted in the United Kingdom have shown that a nitric oxide nasal spray (NONS, SaNOtize) is both a safe and effective antiviral treatment to prevent COVID-19 transmission and symptom duration, as well as reduce symptom severity and damage in those already infected, according to the study authors.
“NONS destroys the virus, blocks entry into and halts viral replication within the nasal cavity, which rapidly reduces viral load. This is significant because viral load has been linked to infectivity and poor outcomes,” said Chris Miller, PhD, RT, chief science officer and co-founder of SaNOtize, in a press release. “There is currently a lack of an antiviral therapy that is effective against COVID-19 and its variants, can prevent or shorten the course of the disease, reduce damage, lower the severity of COVID-19, and can be made widely and readily available to the public.”
Results of clinical trials have shown that a nitric oxide nasal spray is both a safe and effective antiviral treatment to prevent COVID-19 transmission and symptom duration, as well as reduce symptom severity and damage in those already infected.

Horrifying 1914-horsepower AWD system, 0–60 mph will take just 1.85 seconds, and the quarter-mile will take about 8.6 seconds – faster than anything that’s come before.
Rimac is leaving behind the “C_Two” pre-production moniker and charging forward with the production-ready Nevera, a next-level electric hypercar that makes us ask, do we really want to go that fast? Rimac’s latest battery-wired endeavor rockets drivers from 0 to 60 mph in a bowel-loosening 1.85 seconds before flirting with world-record levels of pure, unfiltered speed. The high-tech wonder-car with seven-figure price tag also comes stuffed bumper to bumper with the latest tech, including AI-powered driver-performance assistance, steer-by-wire and second-gen torque vectoring.
Rimac has finally ditched the eyesore Concept_One/Two naming structure for a model name that befits a €2-million electric hypercar. The company explains that “Nevera” comes from the thunderous, high-voltage world of meteorology, a colloquialism that references a sudden and unexpected Mediterranean storm ripping across the Adriatic Sea off the Croatian coast. It’s not hard to see how that name fits like a glove around a bleeding-edge megacar built to rip across asphalt in a way human drivers may or may not be prepared for.
“This is it. This is the car I had in mind when I embarked on the ‘impossible’ journey 10 years ago,” Rimac founder and CEO Mate Rimac proclaims. “When we first revealed the C_Two, we set our targets extremely high. There was nothing else that could even come close to matching the car’s cutting-edge electric powertrain and extreme performance. But for us, that was only the starting point.”
A new discovery could lead to new drugs for faster repairing muscles after injury — or rebuilding muscle mass lost during the normal aging process.
Researchers at the Salk Institute have uncovered a mechanism by which stem cells can help regenerate muscles. The discovery could provide a new drug target for repairing muscles after injury or rebuilding muscle mass lost during the normal aging process.
The breakthrough started with a set of proteins called Yamanaka factors, which have long been studied as a key part of stem cell therapy. These factors are used to convert regular cells – most commonly skin cells – into what are known as induced pluripotent stem cells (iPS), which can then go on to differentiate into a variety of other cell types. That in turn helps regenerate tissue. But exactly how the Yamanaka factors worked their magic remained a mystery.
“Our laboratory previously showed that these factors can rejuvenate cells and promote tissue regeneration in live animals,” says Chao Wang, first author of the study. “But how this happens was not previously known.”

A first-in-human, Phase 1 trial assessing the safety and immunogenicity of an investigational nanoparticle influenza vaccine designed to provide long-lasting protection against multiple flu virus strains has begun at the National Institutes of Health Clinical Center in Bethesda, Maryland. Healthy participants 18 to 50 years old will receive either a licensed seasonal influenza vaccine or the experimental vaccine, FluMos-v1. Scientists from NIH’s National Institute of Allergy and Infectious Diseases (NIAID) developed FluMos-v1 to stimulate antibodies against multiple influenza virus strains by displaying part of the influenza virus hemagglutinin (HA) protein on self-assembling nanoparticle scaffolds. Alicia T. Widge, M.D., of NIAID’s Vaccine Research Center (VRC), is the principal investigator of the NIAID-sponsored single-site trial.
“The health and economic burdens of influenza are substantial, and the world badly needs improved flu vaccines,” said NIAID Director Anthony S. Fauci, M.D. “I am encouraged by the great promise of the VRC nanoparticle vaccine candidate, which so far has performed very well in pre-clinical testing.”
Standard influenza vaccines must be reformulated and administered annually to match changes in the HA protein in the viral strains predicted to dominate in the upcoming influenza season. If the vaccine is not well matched to dominant circulating virus strains, the antibodies elicited may provide sub-optimal protection. So-called universal influenza vaccines are being developed and tested by many research groups and could one day eliminate the need for annual vaccination by generating long-lasting antibodies to protect against many existing or emergent influenza virus strains, including those not represented in the vaccine.