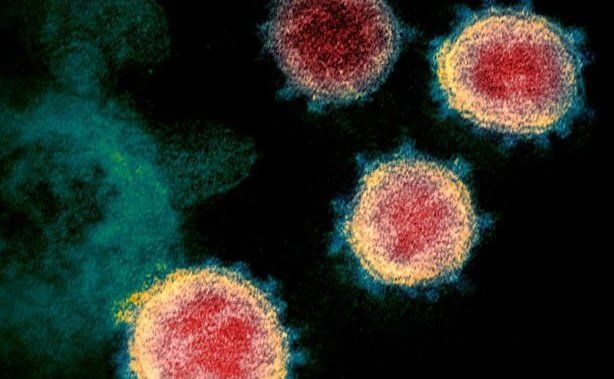
The Delta B.1.617 variant has been suggested to be more transmissible than other variants of concern, and has already spread to all ten provinces and one territory.

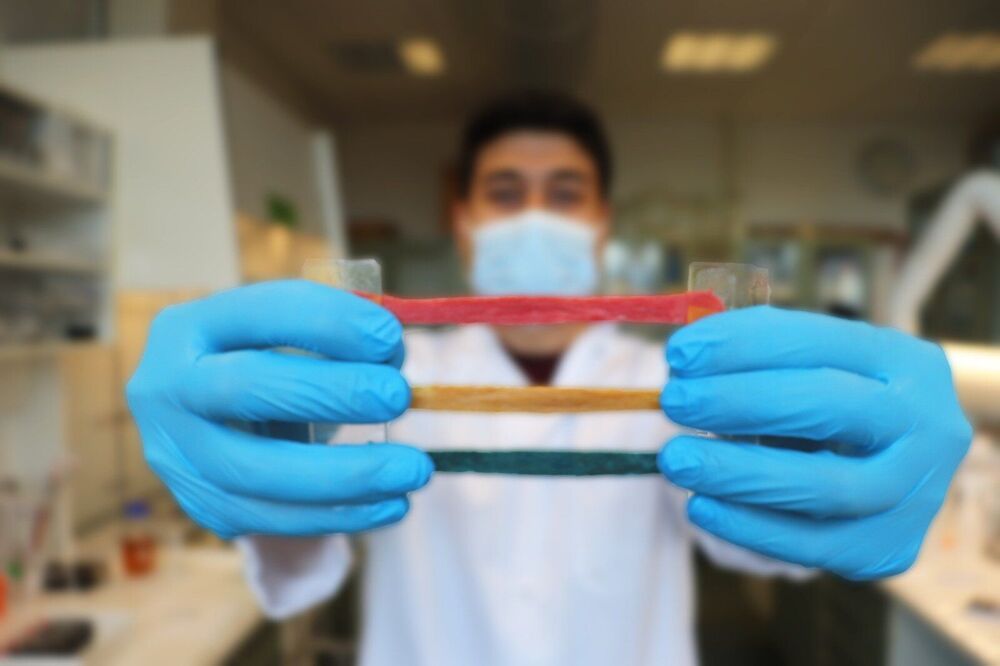
Researchers at DTU Health Tech have developed a new material that can facilitate a near-perfect merger between machines and the human body for diagnostics and treatment.
A DTU research team consisting of Malgorzata Gosia Pierchala, Firoz Babu Kadumundi, and Mehdi Mehrali from #TeamBioEngine headed by Alireza Dolatshahi-Pirouz, have developed a new material—CareGum—that among other things has potential for monitoring motor impairment associated with neurological disorders such as Parkinson’s.

Don’t worry you haven’t stumbled onto that strange part of the internet again, but it is true that we never truly did sequence the entire Human genome. For you see what was completed in June 2000 was the so called ‘first draft’, which constituted roughly 92% of genome. The problem with the remaining 8% was that these were genomic ‘dead zones’, made up of vast regions of repeating patterns of nucleotide bases that made studying these regions of the genome effectively impossible with the technology that was available at the time.
However, recent breakthroughs in high throughput nanopore sequencing technology have allowed for these so call dead zones to be sequences. Analysing these zone revealed 80 different genes which had been missed during the initial draft of the Human genome. Admittedly this is not many considering that the other 92% of the genome contain 19889 genes, but it may turn out that these genes hold great significance, as there are still many biological pathways which we do not fully understand. It is likely that many of these genes will soon be linked with what are known as orphan enzymes, which are proteins that are created from an unidentified gene, which is turn opens up the door to studying these enzymes more closely via controlling their expression.
So how does this discovery effect the field of regenerative medicine? Well the discovery of these hidden genes is potentially very significant for our general understand of Human biology, which in turn is important for our understanding of how we might go about fixing issues which arise. Possibly more important that the discovery of these hidden genes, is the milestone this sequencing represents in our ability to study our genomes quickly and efficiently with an all-inclusive approach. The vast amount of data that will soon be produced via full genome analysis will go a long way towards understanding the role that genetics play in keeping our bodies healthy, which in turn will allow us to replicate and improve upon natural regenerative and repair mechanisms. It might even allow us to come up with some novel approaches which have no basis in nature.

When attacked by chemotherapy, all cancer cells have the ability to start hibernating in order to wait out the threat, finds new research.
Researchers discover that cancer cells go into hibernation to avoid chemotherapy effects.
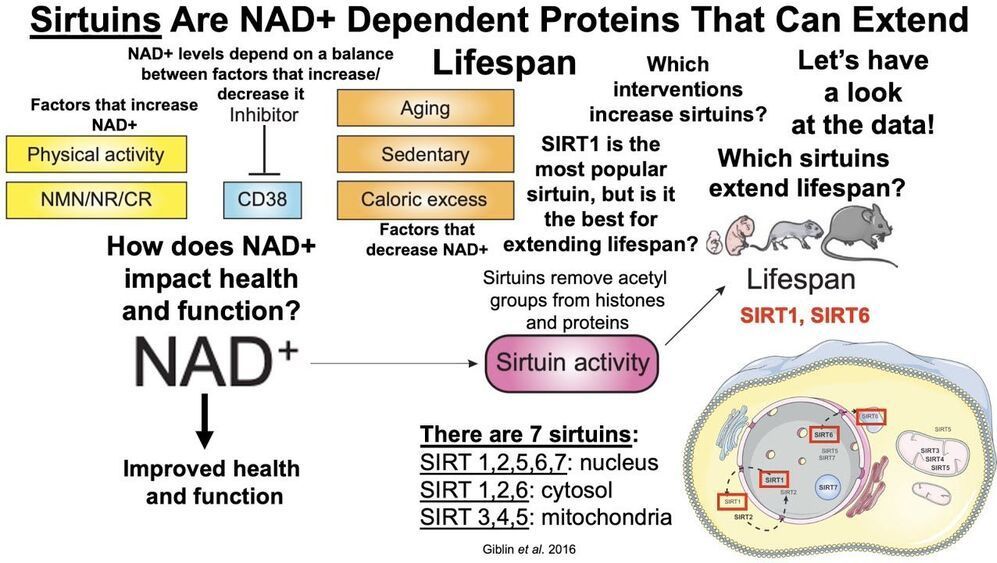
Papers referenced in the video:
Sirtuins, Healthspan, and Longevity in Mammals.
https://www.sciencedirect.com/science/article/pii/B9780124115965000034
Sirt1 extends life span and delays aging in mice through the regulation of Nk2 homeobox 1 in the DMH and LH
https://pubmed.ncbi.nlm.nih.gov/24011076/
Resveratrol improves health and survival of mice on a high-calorie diet.
https://pubmed.ncbi.nlm.nih.gov/17086191/
Rapamycin, But Not Resveratrol or Simvastatin, Extends Life Span of Genetically Heterogeneous Mice.
https://pubmed.ncbi.nlm.nih.gov/20974732/
Sirt1 improves healthy ageing and protects from metabolic syndrome-associated cancer.
https://www.nature.com/articles/ncomms1001

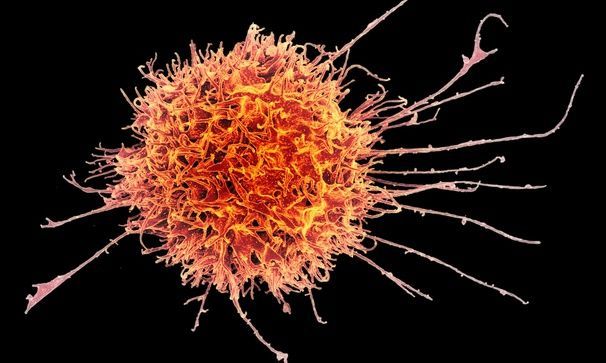
Fate Therapeutics on Thursday reported new results from two early-stage studies testing two types of experimental leukemia treatments that use natural killer cells, an emerging form of cancer immunotherapy.
Research into NK cell treatments remains early, and the field has significant hurdles still to overcome, like proving how potent their effects are and how long they last. It’s unclear what role they’ll play in cancer care. But encouraging signs are emerging, most notably from a lymphoma treatment developed by the MD Anderson Cancer Center.
The field’s progress has led to the launch of multiple startups and elevated the profile of biotechs like Fate and Nkarta Therapeutics, the most advanced, publicly traded companies developing the technology. NK cells are “becoming a very important tool and cell type within this fight against cancer,” said CRISPR Therapeutics CEO Sam Kulkarni in an interview after the biotech formed a broad partnership with Nkarta last week.
Both Fate and Nkarta have begun with acute myeloid leukemia, for which there is a history of “naked,” or non-engineered donor, NK cells being successfully used to treat patients with the disease. The biotechs aim to prove engineered versions that are mass-produced as “off-the-shelf” therapies can be just as effective or better than NK cell transplants.

Scientists have been aware of the existence of stem cells since the 1900’s, but it wasn’t until the turn of the millennium that the medical community (and in turn the public) sat up and took notice of their potential. Unfortunately, the first public debut of stem cell therapy in the eyes of the public was through the political and moral minefield of deriving stem cell lines from human embryos. It was long before religious and secular objections lead to President Bush Banning any Federal funding for studies utilising newly created stem cell lines. The public opinion of stem cells was extremely polarised, with the public split heavily down the middle, between support and condemnation. What happened next was unfortunately as predictable as the tide coming in.
To compensate for the pushback against stem cell research, more and more extravagant claims were made in support of stem cells. Although most of these claims were based upon perfectly reasonable extrapolation from what was known of the potential for stem cells, the time frame in which these advances could be made was wildly underestimated. Confounding that problem was the fact that it would be many years until a method through which stem cells could be reverse engineered from a patient’s tissue, which meant that medical treatments had to based around stem cell lines derived from embryonic stem cell lines, which as discusses previously was an ethical nightmare, as well as being logically untenable for the majority of people (as most people don’t have embryonic tissue samples stored away for future use). Great promises were made to the public, without a full understanding of what was needed in order to get stem cell therapy to a functional level.
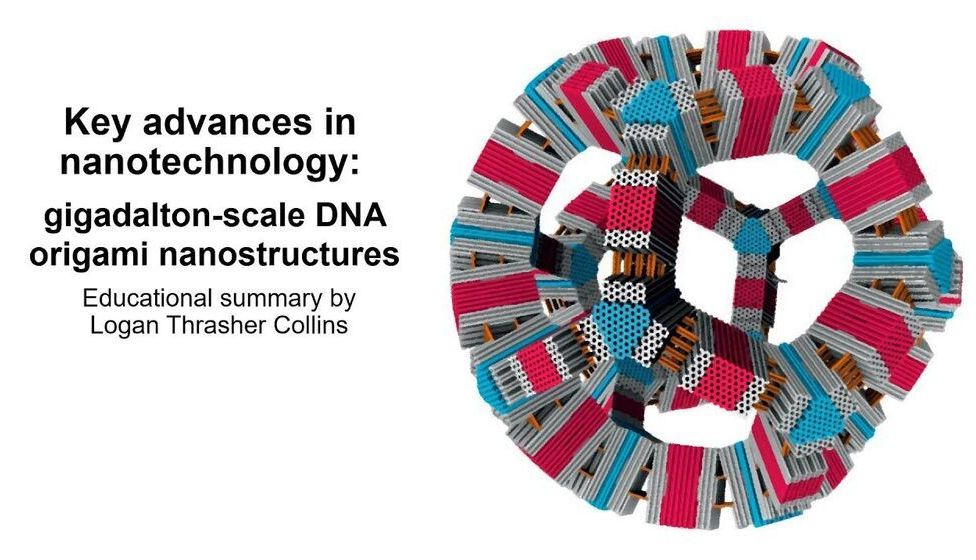
Check out this short educational video in which I explain some super exciting research in the area of nanotechnology: gigadalton-scale DNA origami! I specifically discuss a journal article by Wagenbauer et al. titled “Gigadalton-scale shape-programmable DNA assemblies”.
Here, I explain an exciting nanotechnology paper “Gigadalton-scale shape-programmable DNA assemblies” (https://doi.org/10.1038/nature24651).
Though I am not involved in this research myself, I have worked in adjacent areas such as synthetic biology, nanotechnology-based tools for neuroscience, and gene therapy. I am endlessly fascinated by DNA origami and would love to use it in my own research at some point in the future.
I am a PhD candidate at Washington University in St. Louis and the CTO of the startup company Conduit Computing. I am also a published science fiction writer and a futurist. To learn more about me, check out my website: https://logancollinsblog.com/.
