Category: biotech/medical
The inoculation, called V920, was developed by Merck.
- By Helen Branswell, STAT on May 14, 2018
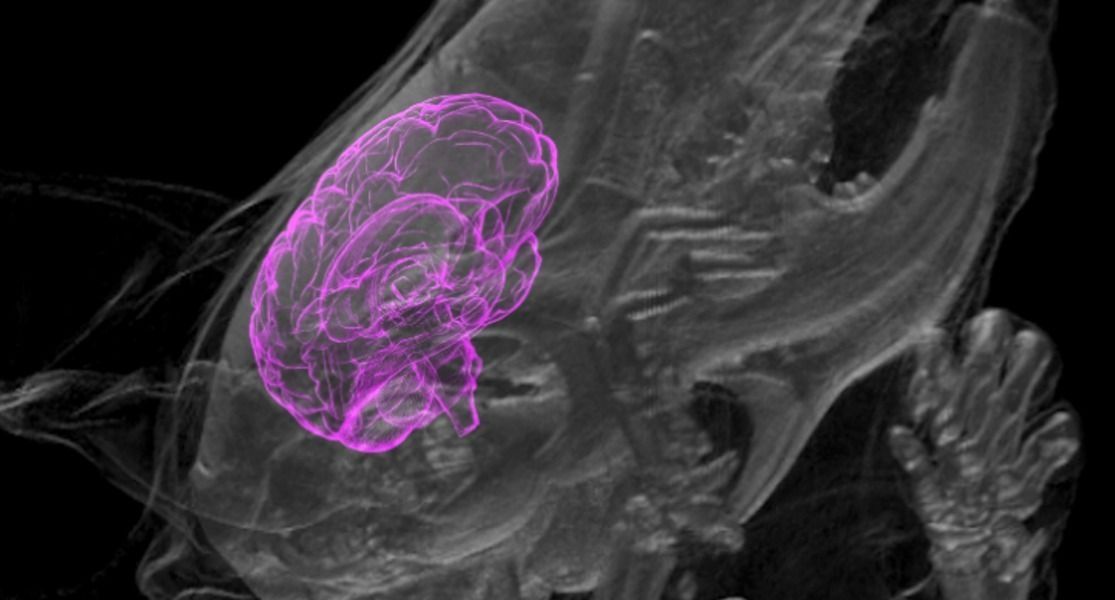
Stem cell technology has advanced so much that scientists can grow miniature versions of human brains — called organoids, or mini-brains if you want to be cute about it — in the lab, but medical ethicists are concerned about recent developments in this field involving the growth of these tiny brains in other animals. Those concerns are bound to become more serious after the annual meeting of the Society for Neuroscience starting November 11 in Washington, D.C., where two teams of scientists plan to present previously unpublished research on the unexpected interaction between human mini-brains and their rat and mouse hosts.
In the new papers, according to STAT, scientists will report that the organoids survived for extended periods of time — two months in one case — and even connected to lab animals’ circulatory and nervous systems, transferring blood and nerve signals between the host animal and the implanted human cells. This is an unprecedented advancement for mini-brain research.
“We are entering totally new ground here,” Christof Koch, president of the Allen Institute for Brain Science in Seattle, told STAT. “The science is advancing so rapidly, the ethics can’t keep up.”
In a paper published Monday in the journal Proceedings of the National Academy of Sciences, researchers demonstrated that the virus could infect human cells as well as the cells of cats and chickens. Even though PDCoV appears to be limited to pigs at the moment, scientists suspect that its sudden appearance in 2012 occurred as a result of a rapid “host switching” event in which the virus adapted to infect pigs, possibly from birds. It causes diarrhea and vomiting in pigs and can be fatal, especially in nursing young. The virus’s probable history, coupled with some specific aspects of how it infects cells, has scientists worried that it could become a threat to human health.
Article continues below.
An interview on rejuvenation science, advocacy, and more with Reason from the blog Fight Aging!.
Most people interested in rejuvenation and life extension are familiar with Fight Aging!, one of the very first rejuvenation advocacy blogs dating back all the way to the early 2000s; if you’re one of them, then you certainly are familiar with Reason, the man behind FA!.
Over the years, Reason has been a patient yet relentless advocate, acting not only as an information provider for the public but also helping out innumerable organizations and companies in the field of rejuvenation biotechnology in financial and other ways. Back in the day when SRF didn’t exist yet, Reason was a volunteer for Methuselah Foundation; eventually, he helped fund companies such as Oisìn Biotechnologies, CellAge, and LysoCLEAR; and, earlier this month, Reason and Bill Cherman co-founded Repair Biotechnologies, a company focused on gene therapy for rejuvenation, as announced on FA!.
Bill Cherman is an investor in the rejuvenation community who, just like Reason, has contributed to development of many ventures in the field. He is a holder of a gold medal in the Brazilian Mathematics Olympiad, a BA in economics, and a candidate in the Master of Biotechnology Enterprise and Entrepreneurship program at Johns Hopkins. He founded Front Seat Capital, a venture capital firm looking to invest in startups with the potential to change the world.
This is important because “the go-to treatment for many cases of depression is medication…this treatment option can cause as many issues as the problem it is trying to solve. Antidepressants can put residents at greater risk of falls, negative health complications and other poor conditions…studies indicate that antidepressants may not be effective for most older Americans. (Additionally) Medication adherence is another significant challenge.”
___ Why technology — not medication — is the future of treating older adults with depression (McKnight’s Long-term Care News): “The go-to treatment for many cases of depression is medication. Unfortunately, this treatment option can cause as many issues as the problem it is trying to solve. Antidepressants can put.
DALLAS – March 29, 2018 – Researchers from UT Southwestern’s Charles and Jane Pak Center for Mineral Metabolism and Clinical Research and Internal Medicine’s Division of Nephrology recently published work in Nature that reveals the molecular structure of the so-called “anti-aging” protein alpha Klotho (a-Klotho) and how it transmits a hormonal signal that controls a variety of biologic processes. The investigation was performed in collaboration with scientists from New York University School of Medicine and Wenzhou Medical University in China.
Studies at UTSW two decades ago by Dr. Makoto Kuro-o, Professor of Pathology, demonstrated that mice lacking either a-Klotho or the hormone FGF23 suffered from premature and multiple organ failure as well as other conditions, including early onset cardiovascular disease, cancer, and cognitive decline. Because defects in a-Klotho lead to symptoms seen in aging, researchers inferred that a-Klotho suppresses aging, leading to great interest in how the a-Klotho protein might work together with the hormone FGF23 to fulfill their roles.
A-Klotho can exist on the surface of a cell or can be released from the cell and circulate in body fluids, including the blood, as soluble a-Klotho. The cell-attached form and the circulating form of a-Klotho were previously and universally believed to serve completely different functions.
While you can’t teach an old dog new tricks, you may be able to make your pup young again.
A Harvard startup has begun preliminary experiments on beagles and claims it can make animals ‘younger’ by adding new DNA instructions to their bodies.
And, the firm says it could one day work for humans, too.
And inflammation is one of the three primary ageing processes.
Scientists have discovered a new metabolic process in the body that can switch off inflammation. They have discovered that ‘itaconate’—a molecule derived from glucose—acts as a powerful off-switch for macrophages, which are the cells in the immune system that lie at the heart of many inflammatory diseases including arthritis, inflammatory bowel disease and heart disease.
The scientists, working in the School of Biochemistry and Immunology in the Trinity Biomedical Sciences Institute at Trinity College Dublin, hope their discovery will have relevance for inflammatory and infectious diseases—and that their findings may also help to develop much-needed new drugs to treat people living with these conditions.
Professor of Biochemistry at Trinity, Luke O’Neill, was, along with Dr. Mike Murphy of the University of Cambridge, the joint leader of the work just published in leading international journal Nature. The discoveries were made using both human cells and mice as a model organism.