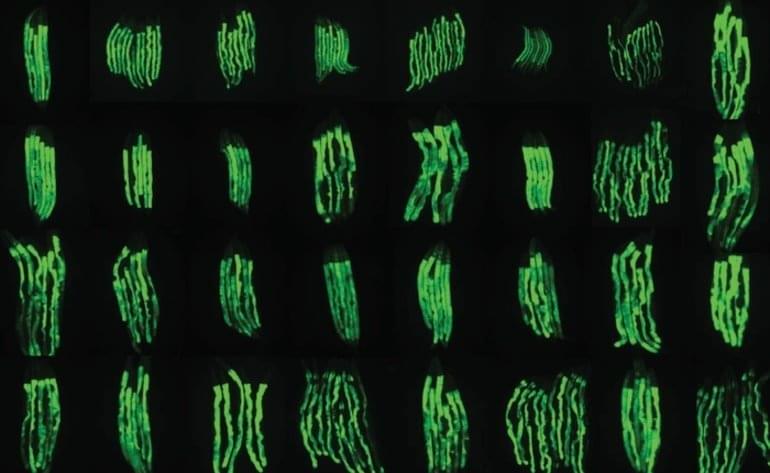
“We think that this indicates that gut bacteria and fungi influence anti-tumor immune responses in many, if not all, types of cancer.”
Cedars-Sinai Cancer researchers have discovered that intestinal microorganisms help regulate anti-tumor immune responses to radiation treatments, and that fungi and bacteria have opposing effects on those responses. The study, conducted in laboratory mice 0 illuminates a path toward improving the effectiveness of radiation and immune-based treatments for patients with melanoma, breast and many other cancers.
The study, published on Aug. 13 in the peer-reviewed journal Cancer Cell, builds on prior studies that focused on the role of intestinal bacteria in influencing immune responses to chemotherapy and immunotherapy. Here the investigators sought to determine what role both bacteria and fungi in the gut might play in the response to radiation therapy.
Trillions of microorganisms live in normal human intestines. These so-called commensal microorganisms are “friendly” bacteria and fungi that help process nutrients and play key roles in regulating the immune system in everything from infections to allergies. The research team found that reducing levels of commensal fungi in the intestines enhanced the anti-tumor immune response in the mice following radiation therapy. Conversely, they showed that depletion of commensal bacteria reduced the anti-tumor response.