https://www.youtube.com/watch?v=Nufc8vTsbBY
Category: bioengineering
New CRISPR-Gold technique reduces behavioral autism symptoms in mice
A remarkable new study has successfully used the CRISPR-Cas9 gene editing technique to edit a specific gene in mice engineered to have fragile X syndrome (FXS), a single-gene disorder often related to autism. The single gene edit in the live mice resulted in significant improvements in repetitive and obsessive behaviors, making this the first time gene editing has been used to effectively target behavioral symptoms related to autism spectrum disorder (ASD).
FXS is a genetic disorder associated with intellectual disability, seizures and exaggerated repetitive behavior. Previous studies have shown that the repetitive behaviors associated with FXS are related to a specific excitatory receptor in the brain that, when dysregulated, causes exaggerated signaling between cells.
The CRISPR technique homes in on the gene that controls that excitatory receptor, the metabotropic glutamate receptor 5 (mGluR5), and essentially disables it, dampening the excessive signaling the corresponds with repetitive behaviors. In mice treated with the new system, obsessive digging behavior was reduced by 30 percent and repetitive leaping actions dropped by 70 percent.
Experimental Drug Injection Causes the Brain to Grow New Neurons
For the first time ever researchers have had a breakthrough in creating a cocktail of drugs that caused new neurons to grow in the brains of mice.
In my last article I gave a detailed account on the debate of neurogenesis. While some neuroscientists claim that neurogenesis takes place within the adult mammalian human brain other researchers contest that idea claiming that new neurons stop developing at a very young age. Whichever side of the debate you are on one thing remains certain, that there are neurological diseases that leave negative impacts on cognitive function. This has left researchers looking for various ways to treat Alzheimer’s, Parkinson’s, and other brain damage.
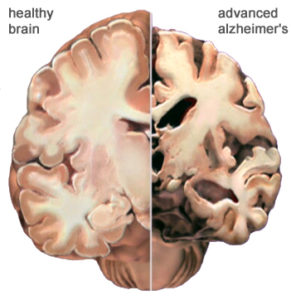
While the brain is incredibly complex and past research has failed to offer much hope for Alzheimer’s disease, Hongkui Deng at Paking University Health Science Center in China may finally be able to change that.
For the first time ever researchers have had a breakthrough in creating a cocktail of drugs that caused new neurons to grow in the brains of mice.
What this cocktail does when injected into the brain is it hijacks the astrocytes into behaving like new neurons. This is significant for a number of reasons. One important detail about astrocyte cells is that they can survive after a stroke while regular neurons die. Another important detail is that there are 10 times more astrocytes in the brain than neurons. That means that there are 1 trillion glia cells within the brain. So not only are they more resilient than neurons they outnumber them too.
Deng and his team of researchers have found that when the cocktail is injected into the brains of mice that it effectively gives the cell a new identity by erasing its old one and giving it a new one. Not only did the cells change shape but they showed change in gene activity too.
The results were substantial. While it remains speculative as to how closely the cells resemble normal neurons, around 80 to 90 per cent of the astrocytes started to resemble neurons and even mimicked their behavior by electrical signals the same way regular neurons do.
“They are unlikely to be a 100 percent match” Deng stated. “But the treatment was safe and none of the mice developed any health problems”.
Matthew Grubb at King’s College London states that “if it holds up it’s absolutely amazing, and has a lot of potential applications and exciting consequences. If you’ve got a degenerating brain, for example in Alzheimer’s disease, and you could get the brain to regrow neurons itself, it would be a huge step forward.”
The next step for the researchers will be to test the cocktail in mice that have had a stroke. The hope is that the cocktail will cause the astrocytes to behave as neurons and help the mice recover.
While Grubb admits it is difficult to predict the effects of the treatment in humans, if it works in mice then it offers new hope for those who suffer from neurodegenerative diseases such as Alzheimer’s or Parkinson’s. While it is unlikely to bring back lost memories Grubb thinks it might restore the ability to create new ones.
Even though the research is promising there still remain challenges as Roger Barker at the University of Cambridge points out. The sheer numbers of cells lost in a neurodegenerative disease is something to consider. “In Parkinson’s, a quarter of a million cells are lost from either side of the brain. Currently Barker is conducting clinical trials of implants of brain tissue taken from aborted fetuses as a treatment for Parkinson’s.
Another challenge is to distinguish the types of neurons the drugs will make. As Grubb points out, if you make too many of the type of neurons that excites their neighbors you end up triggering epilepsy. Grubb also points out that different brain disorders effects different types of neurons which is another reason why we need to be capable of distinguishing them. The neurons that die from Parkinson’s are the neurons that create the chemical dopamine for example.
Another example is balancing the risks for rewards. As Grubb points out “you’d have to have extremely good control over what cells you’re programming, where they’re going to go, and which cells they’ll connect to. If the treatment were to be used to boost grey matter, for example, this could provide a way to improve skills like memory. However, too much grey matter has been linked to causing people to be being easily distracted.
While the research is definitely groundbreaking it is still in its early stages. Hopefully in the near future it will offer treatments to those who need them.

Thousands of Swedes are inserting microchips into themselves – here’s why
Often, different biohacking scenes reflect the different societies and cultures in which they develop. So, for example, European biohackers generally differ from their North American counterparts. North American groups are concerned with developing alternatives to the established healthcare practices. European groups, meanwhile, are more focused on finding ways of helping people in developing countries or engaging in artistic bio-projects.
Sweden’s deep relationship with digital technology helps explain why its biohacking scene is so unique.
New platform will help create designer human proteins in the lab
A group of researchers from Yale University and Agilent Technologies have developed a #syntheticbiology technique that turns bacterium E. Coli into a phosphorylated protein factory capable of churning out every known instance of this modification in human proteins.
Proteins, the end product of genes, carry out life functions. Most human proteins are modified by a process called serine phosphorylation — a chemical switch that can alter their structure and function. Malfunctions in this process have been implicated in diseases such as cancer and Alzheimer’s yet are difficult to detect and study. A group of researchers from Yale University and Agilent Technologies have developed a synthetic biology technique that turns bacterium E. Coli into a phosphorylated protein factory capable of churning out every known instance of this modification in human proteins.
“We synthesized over 110,000 phosphoproteins from scratch and we can now study how they all function together,” said Jesse Rinehart, associate professor of cellular and molecular physiology at the Systems Biology Institute and senior author of the research. “This is the future of scientific research — we can build everything we study.”
Previously, researchers were only able to create a single phosphoprotein at a time. The new platform will help scientists create designer proteins by studying the impact of phosphorylation on all potential protein interactions, the authors say. “Biologists want to know which proteins interact with each other because diseases can arise when these interactions go wrong,” said Karl Barber, a Yale graduate student who is the first author on the study and a recently named Schmidt Science Fellow.
Tissue-engineered human pancreatic cells successfully treat diabetic mice
Researchers tissue-engineered human pancreatic islets in a laboratory that develop a circulatory system, secrete hormones like insulin and successfully treat sudden-onset type 1 diabetes in transplanted mice.
In a study published by Cell Reports, the scientists use a new bioengineering process they developed called a self-condensation cell culture. The technology helps nudge medical science closer to one day growing human organ tissues from a person’s own cells for regenerative therapy, say study investigators at Cincinnati Children’s Hospital Medical Center in the U.S. and Yokohama City University (YCU) in Japan.
“This method may serve as a principal curative strategy for treating type 1 diabetes, of which there are 79,000 new diagnoses per year,” said Takanori Takebe, MD, a physician-scientist at the Cincinnati Children’s Center for Stem Cell and Organoid Medicine. “This is a life-threatening disease that never goes away, so developing effective and possibly permanent therapeutic approaches would help millions of children and adults around the world.”
Harvard Rewinds the Biological Clock of Time
Investigators at Harvard Medical School have identified the key cellular mechanisms behind vascular aging and its effects on muscle health, and they have successfully reversed the process in animals.
The scientists used a chemical compound that’s an NAD+ booster called NMN which plays a critical role in repairing cellular DNA as well as maintaining cell vitality to test what would happen.
Could reversing the aging of blood vessels hold the key to restoring youthful vitality? If the old adage “you are as old as your arteries” reigns true then the answer is yes, at least in mice.
According to a new study by Harvard Medical School researchers, they have identified the cellular mechanisms that cause the aging of vascular arteries as well as the effects of such aging on the health of muscles. The Medical team was also able to successfully reverse this aging process.
What these findings seem to indicate is that there’s a glitch in the normal crosswalk between both muscles and blood vessels and keeping both tissues healthy. The scientists were also able reverse the demise of blood vessels and muscle atrophy in the aging mice by using the synthetic precursors of two molecules naturally present in the body. This boosted their exercise endurance in the process.
The Medical team is excited because such a breakthrough will now pave the way to identifying new therapies for humans.
Study senior investigator David Sinclair, professor in the Department of Genetics at Harvard Medical School and co-director of the Paul F. Glenn Center for the Biology of Aging at Harvard Medical School stated “we’ve discovered a way to reverse vascular aging by boosting the presence of naturally occurring molecules in the body that augment the physiological response to exercise.”
Because there are some very important differences in biology between humans and mice there’s a possibility that this treatment may not have the same effect in humans. Nonetheless, the research team plans to follow through with human clinical trials because the results of this experiment were important enough to prompt the research team in doing so.
Sinclair, who is also a professor at the University of New South Wales School of Medical Sciences in Sydney, Australia stated, “the approach stimulates blood vessel growth and boosts stamina and endurance in mice and sets the stage for therapies in humans to address the spectrum of diseases that arise from vascular aging.”
One of the side effects of aging is reduced blood flow and the compromise of oxygenation of organs and tissue because our tiniest blood vessels began to wither and die. Cardiac and neurologic conditions, muscle loss, impaired wound healing and overall frailty, and among other things are the results of vascular aging. As these blood vessels die there’s a loss of blood flow to organs and tissues which causes toxins build-up and a loss of oxygen.
For quite some time scientists have known the essential role that endothelial cells, which line blood vessels, play in the health and growth of blood vessels that supply oxygen-rich and nutrient-loaded blood to organs and tissues. Unfortunately, as with all things on the human body, these endothelial cells age having a detrimental effect on the body. New blood vessels fail to form, blood vessels atrophy, and the overall blood flow to most parts of the body diminishes. This has a powerful impact on muscles, which heavily rely on robust blood supply to function because they’re heavily vascularized.
Typically we exercise in hopes of slowing down sarcopenia, but unfortunately even that doesn’t last forever. Gradually our muscles grow weaker and begin to shrivel as part of the aging process.
What precisely curtails the blood flow and precipitates this unavoidable decline? Why does even exercise lose its protective power to sustain muscle vitality? Is this process reversible? There were some of the leading questions Sinclair and team had.
The Experiment/Results:
Sinclair and his team discovered through a series of experiments that the flow of blood is reduced as endothelial cells start to lose a critical protein known as sirtuin1, or SIRT1. SIRT1 delays aging and extends life in yeast and mice as shown in previous studies.
Research done previously by Sinclair and others has shown that NAD+ boosts the activity of SIRT1. SIRT1 loss is a result of the loss of NAD+, which is a key regulator of protein interactions and DNA repair that was identified more than a century ago. As NAD+ declines with age so does the protein SIRT1.
The results showed that the critical interface that enables the conversation between endothelial cells in the walls of blood vessels and muscle cells is provided by NAD+ and SIRT1.
SIRT1 signaling is activated and generates new capillaries, the tiniest blood vessels in the body that supply oxygen and nutrients to tissues and organs in young mice muscles. As the mice aged, however, the study found that muscle tissue was left nutrient-deprived and oxygen-starved as a result in the diminishment of NAD+ and SIRT1.
The researchers hope that their findings may pave the way to therapeutic advances that hold promise for the millions of older people for whom regular physical activity is not an option.
Abhirup Das, the studies first author, who conducted the work as a post-doctoral fellow in Sinclair’s lab, currently a visiting scholar in genetics at Harvard Medical School and a post-doctoral research fellow at the University of South New Wales School of Medical Sciences, said that “we reasoned that declining NAD+ levels reduce SIRT1 activity and thus interfere with aging mice’s ability to grow new blood vessels.”
The researchers then set their sights on the NAD+, which is a critical coenzyme for enzymes that fuel reduction-oxidation reactions, carrying electrons from one reaction to another, and as a cosubstrate for other enzymes such as the sirtuins and poly(adenosine diphosphate-ribose) polymerases.
The scientists used a chemical compound that’s an NAD+ booster called NMN (no not m&m!) which plays a critical role in repairing cellular DNA as well as maintaining cell vitality to test what would happen.
One of the results showed that treatment from NMN caused endothelial cells from humans and mice to have strong growth capacity and reduced cell death.
The team then wondered what would happen to a group of mice that were 20 months old—the rough equivalent of 70 in human years given NMN. After a 2 month time span the results showed that NMN treatment restored the number of blood capillaries and capillary density to those seen in younger mice. Blood flow to the muscles also increased and was significantly higher than blood supply to the muscles seen in same-age mice that didn’t receive NMN.
That wasn’t the most surprising result to the researchers however. What they discovered was that the aging mice showed in comparison to the entreated mice that they regained the capacity to exercise by 56 and 80% more. The untreated mice could only run 240 meters, or 780 feet, on average whereas the mice treated with NMN could run 430 meters, or about 1,400 feet, on average. This treatment could be an answer to humans who have lost the capacity to exercise due to other disabilities or age-related diseases.
The next step for the researchers was to explore methods for boosting the activity of SIRT1. To do this the researchers added a second compound NaHS, sodium hydrosulfide, which is known to be a precursor of SIRT1.
For four weeks a group of mice that were 32-month-old mice—the rough equivalent to 90 in human years—receiving the combo treatment. The results were significant! Not only were the mice able to run longer and faster but they were able to outperform the untreated mice by a longshot. The treated mice ran 1.6 times further than the untreated mice.
Study co-author James Mitchell, associate professor of genetics and complex diseases at the Harvard T. H. Chan School of Public Health stated that “these are really old mice so our finding that the combo treatment doubles their running capacity is nothing short of intriguing.”
“This observation underscores the notion that age plays a critical role in the crosstalk between blood vessels and muscles and points to a loss of NAD+ and SIRT1 as the reason behind loss of exercise effectiveness after middle age,” Das said.
One of the ultimate goals for the team is to eventually move forward in developing small-molecule, NMN-based drugs that mimic the effects of exercise—enhanced blood flow and oxygenation of muscles and other tissues. Though they must first replicate their findings first. Such therapies could potentially help with new vessel growth of organs that suffer tissue-damaging loss of blood supply and oxygen, a common scenario in heart attacks and ischemic strokes, the team said.

Major research reveals CRISPR gene-editing could increase cancer risk in cells
Two recently published studies are raising new concerns that the breakthrough CRISPR-Cas9 gene editing system could potentially trigger an increased cancer risk in cells edited using the technique. With human trials using the gene-editing technique set to commence this year, the scientists behind these new studies urge researchers to be aware of this newly discovered and dangerous cancer-driving mechanism.
It has been less than a decade since the revolutionary CRISPR-Cas9 gene-editing technique was discovered, allowing scientists an unprecedented way to accurately edit DNA. For the most part, the technique has proved promising, safe and effective. Last year, a controversial study was published claiming the technique could introduce unintended, off-target mutations, but after a flurry of criticism attacking the veracity of the work it was ultimately retracted.
These two new studies raise entirely new concerns regarding the technique’s potential for triggering cancer in edited cells. One study comes from a collaboration between the University of Cambridge and the Karolinska Institutet, while the other is led by a team of researchers at pharmaceutical company Novartis.
Starch can replace normal plastic in food packaging
Eventually all petroleum-based material in food packaging will have to be replaced with bio-based material. Research done at Karlstad University shows that a mixture of starch and other polymers forms an equally effective protective barrier.
“Food packaging has to protect and extend the shelf life of food, and should also work during transport,” says Asif Javed, doctor in Chemical Engineering at Karlstad University. “To meet these demands, a protective barrier is needed in paper-based packing such as those used for juice or dairy.”