Cyrus Biotechnology in Seattle and Broad Institute of MIT and Harvard launch collaboration to develop optimized CRISPR gene technology.
Cyrus Biotechnology, Inc. Lucas Nivon, 206−258−6561 [email protected]
Cyrus Biotechnology in Seattle and Broad Institute of MIT and Harvard launch collaboration to develop optimized CRISPR gene technology.
Cyrus Biotechnology, Inc. Lucas Nivon, 206−258−6561 [email protected]
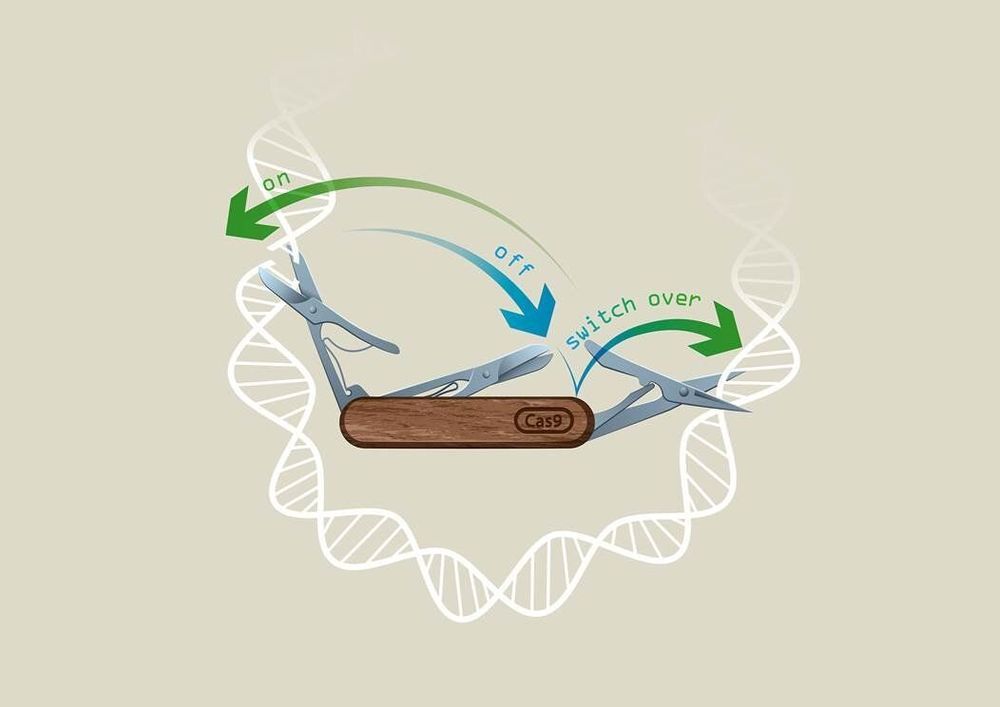
Researchers in Vienna from Ulrich Elling’s laboratory at IMBA—Institute of Molecular Biotechnology of the Austrian Academy of Sciences—in collaboration with the Vienna BioCenter Core Facilities have developed a revolutionary CRISPR technology called “CRISPR-Switch,” which enables unprecedented control of the CRISPR technique in both space and time.
CRISPR/Cas9 technology is based on a modified version of a bacterial defense system against bacteriophages. One of the landmark discoveries for this technique in fact was laid in Vienna and published in 2012 in a study co-authored by Emmanuelle Charpentier and VBC Ph.D. student, Krzysztof Chylinski. Due to its power to also edit mammalian genomes, CRISPR/Cas9 has rapidly established itself as the most employed gene editing method in laboratories across the world with huge potential to find its way to the clinics to cure rare disease. Just a week ago, the first success in the treatment of sickle cell anemia was announced.
To control the power of genome editing, several groups have worked on systems to control editing activity. Scientists from the lab of Ulrich Elling at IMBA were now able to gain unprecedented control over sgRNA activity, in a system termed “CRISPR-Switch.” The results are published in the renowned journal Nature Communications.
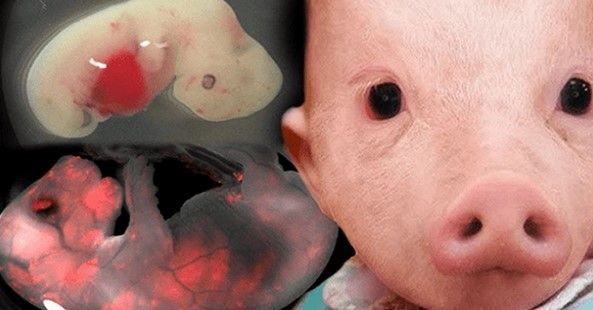
Human-animal hybrids are set to be developed at the University of Tokyo after the Japanese government recently lifted a ban on the controversial stem-cell research.
Hiromitsu Nakauchi—director for Stem Cell Biology and Regenerative Medicine at the University of Tokyo and team leader at Stanford’s Nakauchi Lab—is the first to receive approval for the questionable experiments which will attempt to grow human cells in rat and mouse embryos before being brought to term in a surrogate animal.
Despite many feeling that such studies are the equivalent of playing God, scientists say that the objective is far from sinister. It’s theorized that developing animals with organs constructed from human cells will create organs that can then be used for transplants in humans, cutting the long organ donation waitlists.
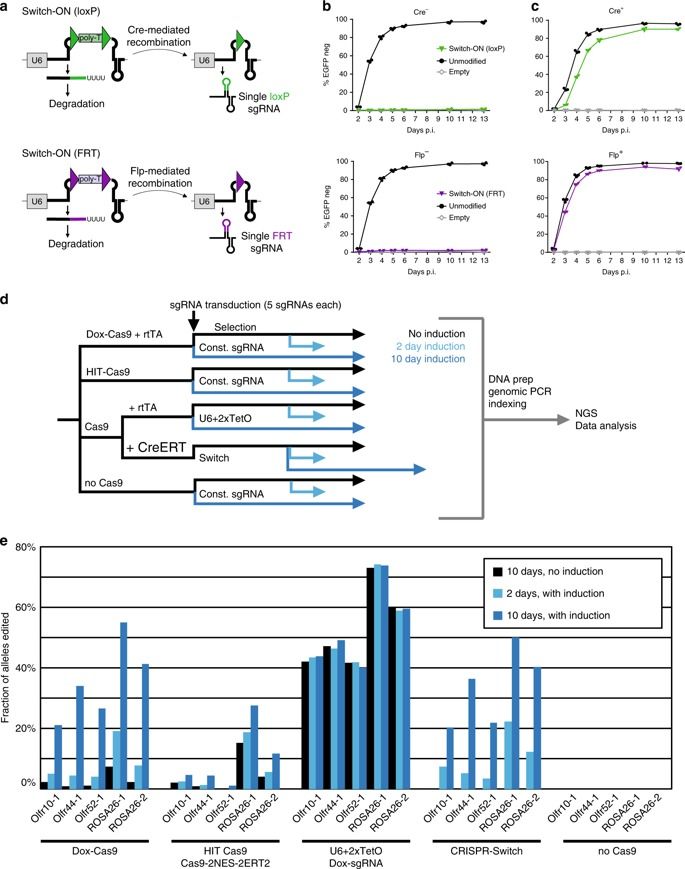
CRISPR-Cas9 is an efficient and versatile tool for genome engineering in many species. However, inducible CRISPR-Cas9 editing systems that regulate Cas9 activity or sgRNA expression often suffer from significant limitations, including reduced editing capacity, off-target effects, or leaky expression. Here, we develop a precisely controlled sgRNA expression cassette that can be combined with widely-used Cre systems, termed CRISPR-Switch (SgRNA With Induction/Termination by Cre Homologous recombination). Switch-ON facilitates controlled, rapid induction of sgRNA activity. In turn, Switch-OFF-mediated termination of editing improves generation of heterozygous genotypes and can limit off-target effects. Furthermore, we design sequential CRISPR-Switch-based editing of two loci in a strictly programmable manner and determined the order of mutagenic events that leads to development of glioblastoma in mice. Thus, CRISPR-Switch substantially increases the versatility of gene editing through precise and rapid switching ON or OFF sgRNA activity, as well as switching OVER to secondary sgRNAs.
Just seven years after scientists announced the first use of Crispr-Cas9 gene editing technology on human cells, researchers shared new evidence this week that Crispr can be used to cure two serious genetic disorders.
On Tuesday, NPR reported that a patient in Nashville had seen a dramatic decline in her symptoms of sickle cell disease after receiving a single gene therapy treatment in July. Sickle cell, which can lead to inflammation, debilitating pain, and life-threatening circulatory problems, affects millions of people around the world.
That same day, the biotech companies behind the sickle-cell treatment, Crispr Therapeutics and Vertex, also shared promising results from their first attempt to cure a case of beta thalassemia, another genetic disorder that affects blood proteins. Nine months after receiving the experimental treatment, a patient in Germany with beta thalassemia has almost no signs of the disorder.

Promising preliminary data from one of the first human trials testing the safety and efficacy of a CRISPR gene therapy has just been revealed. Although it is too early to evaluate long-term effects, the initial reports are impressively successful for two patients with severe genetic blood diseases.
Until February of this year, when pharmaceutical companies CRISPR Therapeutics and Vertex began a large global trial into a treatment called CTX001, no human outside of China had been officially treated with a CRISPR-based gene editing therapy.
CTX001 was developed to treat two types of inherited blood disease, beta-thalassemia and sickle cell disease. Both conditions are caused by a mutation in a single gene and the treatment involves engineering a patient’s stem cells with a single genetic change designed to raise levels of fetal hemoglobin in red blood cells.
