https://www.youtube.com/watch?v=Nufc8vTsbBY
Category: aging
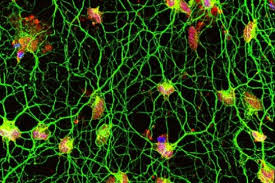
Experimental Drug Injection Causes the Brain to Grow New Neurons
For the first time ever researchers have had a breakthrough in creating a cocktail of drugs that caused new neurons to grow in the brains of mice.
In my last article I gave a detailed account on the debate of neurogenesis. While some neuroscientists claim that neurogenesis takes place within the adult mammalian human brain other researchers contest that idea claiming that new neurons stop developing at a very young age. Whichever side of the debate you are on one thing remains certain, that there are neurological diseases that leave negative impacts on cognitive function. This has left researchers looking for various ways to treat Alzheimer’s, Parkinson’s, and other brain damage.
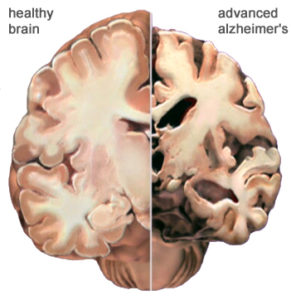
While the brain is incredibly complex and past research has failed to offer much hope for Alzheimer’s disease, Hongkui Deng at Paking University Health Science Center in China may finally be able to change that.
For the first time ever researchers have had a breakthrough in creating a cocktail of drugs that caused new neurons to grow in the brains of mice.
What this cocktail does when injected into the brain is it hijacks the astrocytes into behaving like new neurons. This is significant for a number of reasons. One important detail about astrocyte cells is that they can survive after a stroke while regular neurons die. Another important detail is that there are 10 times more astrocytes in the brain than neurons. That means that there are 1 trillion glia cells within the brain. So not only are they more resilient than neurons they outnumber them too.
Deng and his team of researchers have found that when the cocktail is injected into the brains of mice that it effectively gives the cell a new identity by erasing its old one and giving it a new one. Not only did the cells change shape but they showed change in gene activity too.
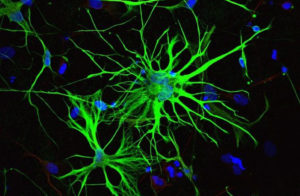
The results were substantial. While it remains speculative as to how closely the cells resemble normal neurons, around 80 to 90 per cent of the astrocytes started to resemble neurons and even mimicked their behavior by electrical signals the same way regular neurons do.
“They are unlikely to be a 100 percent match” Deng stated. “But the treatment was safe and none of the mice developed any health problems”.
Matthew Grubb at King’s College London states that “if it holds up it’s absolutely amazing, and has a lot of potential applications and exciting consequences. If you’ve got a degenerating brain, for example in Alzheimer’s disease, and you could get the brain to regrow neurons itself, it would be a huge step forward.”
The next step for the researchers will be to test the cocktail in mice that have had a stroke. The hope is that the cocktail will cause the astrocytes to behave as neurons and help the mice recover.
While Grubb admits it is difficult to predict the effects of the treatment in humans, if it works in mice then it offers new hope for those who suffer from neurodegenerative diseases such as Alzheimer’s or Parkinson’s. While it is unlikely to bring back lost memories Grubb thinks it might restore the ability to create new ones.
Even though the research is promising there still remain challenges as Roger Barker at the University of Cambridge points out. The sheer numbers of cells lost in a neurodegenerative disease is something to consider. “In Parkinson’s, a quarter of a million cells are lost from either side of the brain. Currently Barker is conducting clinical trials of implants of brain tissue taken from aborted fetuses as a treatment for Parkinson’s.
Another challenge is to distinguish the types of neurons the drugs will make. As Grubb points out, if you make too many of the type of neurons that excites their neighbors you end up triggering epilepsy. Grubb also points out that different brain disorders effects different types of neurons which is another reason why we need to be capable of distinguishing them. The neurons that die from Parkinson’s are the neurons that create the chemical dopamine for example.
Another example is balancing the risks for rewards. As Grubb points out “you’d have to have extremely good control over what cells you’re programming, where they’re going to go, and which cells they’ll connect to. If the treatment were to be used to boost grey matter, for example, this could provide a way to improve skills like memory. However, too much grey matter has been linked to causing people to be being easily distracted.
While the research is definitely groundbreaking it is still in its early stages. Hopefully in the near future it will offer treatments to those who need them.

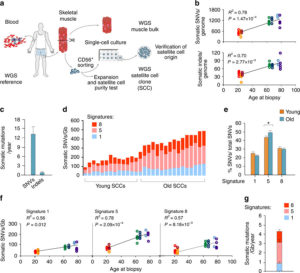
Human Skeletal Muscle Aging and Mutagenesis
Study based upon human skeletal muscle aging, mutagenesis, and the role of #satellite cells.
“A more comprehensive understanding of the interplay of stem cell–intrinsic and extrinsic factors will set the stage for improving cell therapies capable of restoring tissue homeostasis and enhancing muscle repair in the aged.”
Human aging has multiple effects on the human body. One of the effects of human aging is the reduction in skeletal muscle (SkM) function and a reduction in the number and activity of satellite cells (SCs), the resident stem cells. The whole genome of single SC clones of the leg muscle vastus lateralis from healthy individuals of different ages (21–78 years) was analyzed, to study the specific connection between SC aging and muscle impairment. In healthy adult muscle rapid increase of SCs is consistent with the accumulation rate of 13 somatic mutations per genome per year. Mutations typically do not happen in SkM-expressed genes because they are protected. However, as mutations in exons and promoters increase, genes involved in SC activity and muscle function are targeted which results in aging. Exons are coding sections of an RNA transcript, or the DNA encoding it, that are translated into protein. Proteins are the synthesis of molecules. A change in of a single base pair that caused the substitution of a different amino acid in the resulting protein (missense mutation) that was propagated to the muscle and detected in association with SC mutations affecting the whole tissue. #Somatic mutagenesis in SCs as a result is the driving force in the age related decline of SkM function.
Satellite Cells
Satellite cells (SCs) are a heterogeneous population of stem and progenitor cells. These cells play an important role in the growth and development of myofiber. The enlargement, regeneration, and remodeling in skeletal muscle (SkM) is the pivotal role of satellite cells. Satellite cells are dormant until they become activated through exercise or SkM injury. Upon injury skeletal muscle have a remarkable ability to recover from injury. Skeletal muscle goes through a sophisticated degeneration and regenerative process that takes place at the tissue, cellular, and molecular levels. This regenerative process relies upon the dynamic interplay between satellite cells and their environment (stem cell niche). SCs multiply further when committed to myogenic differentiation. As SCs proliferate further they begin to combine with existing SkM fibers and supply new nuclei to the growing and regenerating fibers. The declining of numbers of proliferative potential of SCs is one sign of aging in human SkMs.
A flawed SC compartment is foreseen as a major contributor for age-related deficiencies such as, skeletal muscle tissue having restricted mobility and voluntary functions. The results of such defects include a reduced capacity to respond to hypertrophic stimuli such as exercise and impaired recovery from muscle disuse and injury and the disruption of muscle tissue homeostasis. Moreover, the SCs of nonactive adult animals have been shown to contribute to differentiated fibers in non-injured muscles. Less important is the basal turnover of nuclei in adult fibers in the protection from sarcopenia. This hypothesis was tested and showed that lifelong reduction of satellite cells neither accelerated nor exacerbated sarcopenia and that satellite cells did not contribute to the maintenance of muscle size or fiber type composition during aging, but that their loss may contribute to age-related muscle fibrosis. The progressive loss of SkM mass and function known as sarcopenia affects up to 29% of the population aged 85 years. The accumulation of sarcopenia causes a highly disabling condition. It is essential, nonetheless, that the characterization of SCs in human pathology be further explored. SCs are a key factor in limiting the occurrence of fibrosis in the SkM of mice affected by sarcopenia.
The progressive loss of SkM mass and function known as sarcopenia affects up to 29% of the population aged 85 years. The accumulation of sarcopenia causes a highly disabling condition. It is essential, nonetheless, that the characterization of SCs in human pathology be further explored. Scs are key in limiting the occurrence of fibrosis in the SkM of mice affected by sarcopenia. Genome integrity is essential for the function of stem-cells. But there still must be some stability of the genome. Genetic mutations in the soma has diverse physiological roles and pathological consequences, such as the decline of stem-cell functions. Starting from the first division of the embryo, modifications in the genome extend from single-base changes (single-nucleotide variants (SNVs)) to insertions or deletions of a few bases (indels) to chromosomal rearrangements and occur during the whole life. Somatic variants are not propagated to the whole individual but to a subpopulation of cells in the body, which is strikingly different from germline variants. Adult human tissues become a mosaic of genetically different cells as a result. Furthermore, as a result of the buildup of errors taking place either during cell-division or because of environmental induced DNA damage, somatic mutation burden increases, causing age-related disease. Currently, somatic mutation burden in human SCs or SkM is unknown.
The purpose of the investigation of genetic alterations that occur with aging in the genome of human adult SCs is to use the results to clearly explain mutational processes and SC replication rate occurring in vivo in adult human muscles. The prediction of global consequences on muscle aging and sarcopenia was done by evaluating the functional effects of somatic mutations on SC proliferation and differentiation.
Results
- An accumulation of 13 mutations per genome per year that results in a 2–3-fold higher mutation load in active genes and promoters in aged SCs.
- High mutation burden correlates with defective SC function. • The accumulation of somatic mutations as an intrinsic factor contributing to impaired muscle function with aging.
- The accumulation of somatic mutations as an intrinsic factor contributing to impaired muscle function with aging.
Resources:
“Somatic mutagenesis in satellite cells associates with human skeletal muscle aging.”
Nature Communications volume 9, Article number: 800(2018) Full Abstract Study
“Satellite Cells and the Muscle Stem Cell Niche.”
Physiological Reviews Volume 93, No.1 (2013) Physiological Reviews
“Tissue-specific mutation accumulation in human adult stem cells during life.”
Nature International Journal of Science volume 538, pages 260–264 (13 October 2016) Abstract Study
“When stem cells grow old: phenotypes and mechanisms of stem cell aging”
Development for advances in developmental biology and stem cells Development 2016 143: 314 Abstract Study “Clock-like mutational processes in human somatic cells.”
Nature Genetics volume 47, pages 1402–1407 (2015) Abstract Study

Can You Actually Hack Your DNA to Slow Down Aging? — Bioquark Inc. — Ira Pastor
http://www.thepathmag.com/can-you-actually-hack-your-dna-to-slow-down-aging/
Many technologies / interventions progressing down the development pathways in the coming years — but there are a lot of free, common sense adjustments you can make today:



