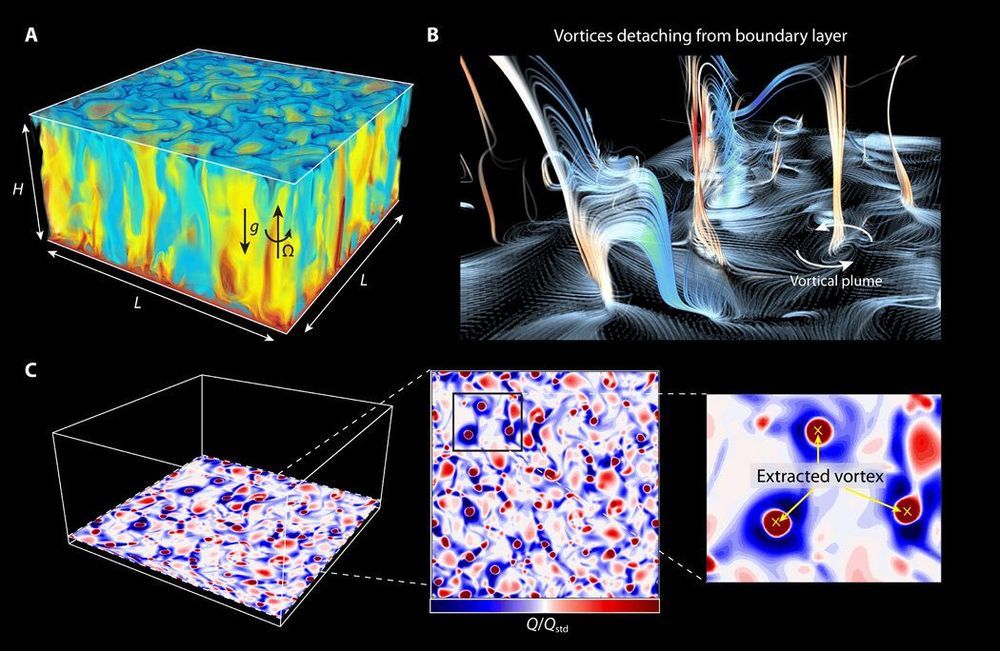
Brownian motion of particles in fluid is a common collective behavior in biological and physical systems. In a new report on Science Advances, Kai Leong Chong, and a team of researchers in physics, engineering, and aerospace engineering in China, conducted experiments and numerical simulations to show how the movement of vortices resembled inertial Brownian particles. During the experiments, the rotating turbulent convective vortical flow allowed the particles to move ballistically at first and diffusively after a critical time in a direct behavioral transition—without going through a hydrodynamic memory regime. The work implies that convective vortices have inertia-induced memory, so their short-term movement was well-defined in the framework of Brownian motion here for the first time.
Brownian motion
Albert Einstein first provided a theoretical explanation to Brownian motion in 1905 with the movement of pollen particles in a thermal bath, the phenomenon is now a common example of stochastic processes that widely occur in nature. Later in 1908, Paul Langevin noted the inertia of particles and predicted that their motion would be ballistic within a short period of time, changing to diffuse motion after a specific timeline. However, due to the rapidity of this transition, it took more than a century for researchers to be able to directly observe the phenomenon. Nevertheless, the “pure” Brownian motion predicted by Langevin was not observed in liquid systems and the transition spanned a broad range of time scales. The slow and smooth transition occurred due to the hydrodynamic memory effect, to ultimately generate long-range correlations.