Sharp wave-ripples from the hippocampus are shown to modulate peripheral glucose homeostasis in rats, offering insights into the mechanism that links sleep disruption and blood glucose regulation in type 2 diabetes.



FENCE program selects researchers to develop low-power, low-latency neuromorphic camera technologies to enable future military applications.
DARPA today announced that three teams of researchers led by Raytheon, BAE Systems, and Northrop Grumman have been selected to develop event-based infrared (IR) camera technologies under the Fast Event-based Neuromorphic Camera and Electronics (FENCE) program. Event-based – or neuromorphic – cameras are an emerging class of sensors with demonstrated advantages relative to traditional imagers. These advanced models operate asynchronously and only transmit information about pixels that have changed. This means they produce significantly less data and operate with much lower latency and power.
“Neuromorphic refers to silicon circuits that mimic brain operation; they offer sparse output, low latency, and high energy efficiency,” said Dr. Whitney Mason, the program manager leading the FENCE program. “Event-based cameras operate under these same principles when dealing with sparse scenes, but currently lack advanced ‘intelligence’ to perform more difficult perception and control tasks.”
Today’s state-of-the-art (SOTA) cameras work well with scenes that have few changes to track and the imagery is relatively simple. Take, for example, a scene of a plane moving through a clear blue sky. SOTA imagers could easily track the movement of the plane. Their capabilities fail, however, in highly cluttered and dynamic scenes, limiting their use among many military applications.

Summary: Young people who experienced complex early life trauma as a result of interpersonal violence or child abuse had more severe mental health problems and cognitive impairments than their peers with no exposure to trauma.
Source: King’s College London.
New research from King’s has explored whether different types of trauma confer the same risk of future mental illness, in the first study of its kind.

Summary: Researchers discuss different current neural network models and consider the steps that need to be taken to make them more realistic, and thus more useful, as possible.
Source: University of Plymouth.
Neuroscience is a field most obviously associated with medicine and/or psychology. However, my background in physics and computer science enables me to explore, and further understand, how the brain computes and stores information, identifying the underlying physical mechanisms and the interplay between them.
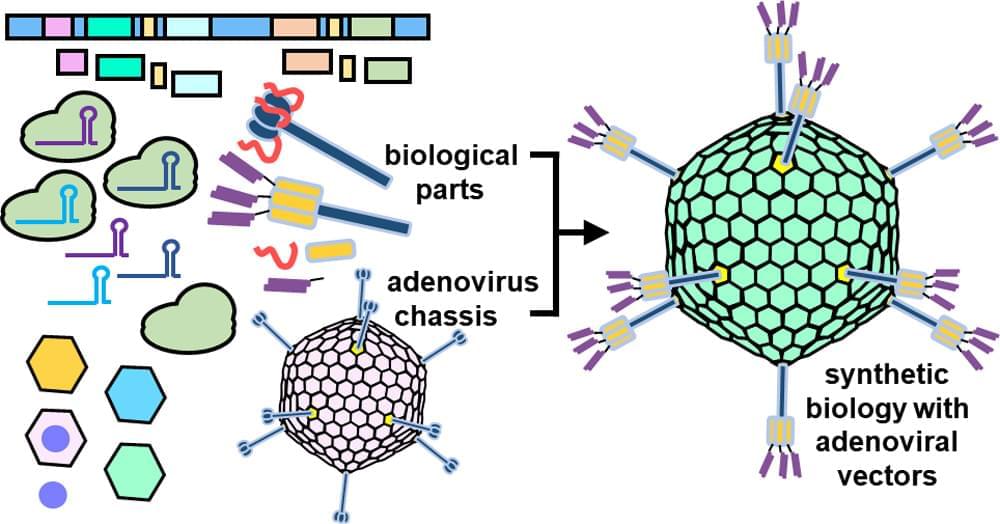
I am pleased to announce that my lead-author review paper has been published in ACS Nano! If you are interested in learning about the convergence of synthetic biology and adenoviral gene therapy, I encourage you to check out my paper.
If you cannot access the full text, I have also posted a local copy at the following link: https://logancollinsblog.files.wordpress.com/2021/08/synthetic-biology-approaches-for-engineering-next-generation-adenoviral-gene-therapies-2021.pdf.
#ACS #ACSNano #SyntheticBiology #GeneTherapy #Biology #Biotech #Science #Biotechnology #Nanotechnology #Adenovirus #Engineering #Virology
Synthetic biology centers on the design and modular assembly of biological parts so as to construct artificial biological systems. Over the past decade, synthetic biology has blossomed into a highly productive field, yielding advances in diverse areas such as neuroscience, cell-based therapies, and chemical manufacturing. Similarly, the field of gene therapy has made enormous strides both in proof-of-concept studies and in the clinical setting. One viral vector of increasing interest for gene therapy is the adenovirus (Ad). A major part of the Ad’s increasing momentum comes from synthetic biology approaches to Ad engineering. Convergence of gene therapy and synthetic biology has enhanced Ad vectors by mitigating Ad toxicity in vivo, providing precise Ad tropisms, and incorporating genetic circuits to make smart therapies which adapt to environmental stimuli. Synthetic biology engineering of Ad vectors may lead to superior gene delivery and editing platforms which could find applications in a wide range of therapeutic contexts.
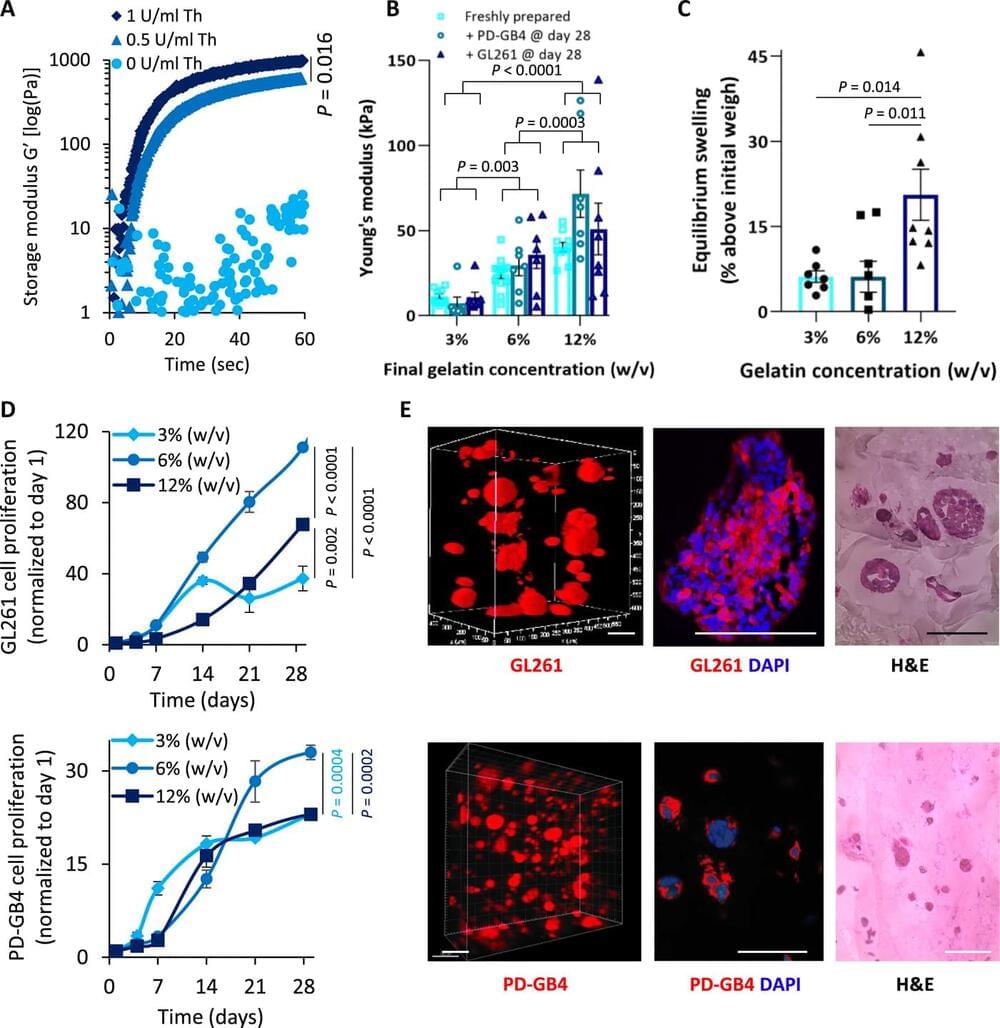
Many drugs show promising results in laboratory research but eventually fail clinical trials. We hypothesize that one main reason for this translational gap is that current cancer models are inadequate. Most models lack the tumor-stroma interactions, which are essential for proper representation of cancer complexed biology. Therefore, we recapitulated the tumor heterogenic microenvironment by creating fibrin glioblastoma bioink consisting of patient-derived glioblastoma cells, astrocytes, and microglia. In addition, perfusable blood vessels were created using a sacrificial bioink coated with brain pericytes and endothelial cells. We observed similar growth curves, drug response, and genetic signature of glioblastoma cells grown in our 3D-bioink platform and in orthotopic cancer mouse models as opposed to 2D culture on rigid plastic plates. Our 3D-bioprinted model could be the basis for potentially replacing cell cultures and animal models as a powerful platform for rapid, reproducible, and robust target discovery; personalized therapy screening; and drug development.
Cancer is the second leading cause of death globally. It is estimated that around 30 to 40% of patients with cancer are being treated with ineffective drugs ; therefore, preclinical drug screening platforms attempt to overcome this challenge. Several approaches, such as whole-exome or RNA sequencing (RNA-seq), aim to identify druggable, known mutations or overexpressed genes that may be exploited as a therapeutic target for personalized therapy. More advanced approaches offer to assess the efficacy of a drug or combinations of drugs in patient-derived tumor xenograft models or in vitro three-dimensional (3D) organoids. Unfortunately, most of the existing methods face unmet challenges, which limit their efficacy. For instance, cells can become quiescent or acquire somatic mutations while growing many generations on plastic under the influence of static mechanical forces and in the absence of functional vasculature.
Investigators who previously developed a recipe for turning skin cells into primitive muscle-like cells that can be maintained indefinitely in the lab without losing the potential to become mature muscle have now uncovered how this recipe works and what molecular changes it triggers within cells. The research, which was led by scientists at Massachusetts General Hospital (MGH) and is published in Genes & Development, could allow clinicians to generate patient-matched muscle cells to help treat muscle injuries, aging-related muscle degeneration, or conditions such as muscular dystrophy.
It’s known that expression of a muscle regulatory gene called MyoD is sufficient to directly convert skin cells into mature muscle cells; however, mature muscle cells do not divide and self-renew, and therefore they cannot be propagated for clinical purposes. “To address this shortcoming, we developed a system several years ago to convert skin cells into self-renewing muscle stem-like cells we coined induced myogenic progenitor cells, or iMPCs. Our system uses MyoD in combination with three chemicals we previously identified as facilitators of cell plasticity in other contexts,” explains senior author Konrad Hochedlinger, Ph.D., a principal investigator at the Center for Regenerative Medicine at MGH and a professor of medicine at Harvard Medical School.
In this latest study, Hochedlinger and his colleagues uncovered the details behind how this combination converts skin cells into iMPCs. They found that while MyoD expression alone causes skin cells to take on the identity of mature muscle cells, adding the three chemicals causes the skin cells to instead acquire a more primitive stem cell–like state. Importantly, iMPCs are molecularly highly similar to muscle tissue stem cells, and muscle cells derived from iMPCs are more stable and mature than muscle cells produced with MyoD expression alone.

1,634 views • Aug 13 2021 • Christof Koch — Allen Institute for Brain Science, Tiny Blue Dot Foundation.