Credit: Dani Clode. #Neuroscience #Research #Biology #Technology
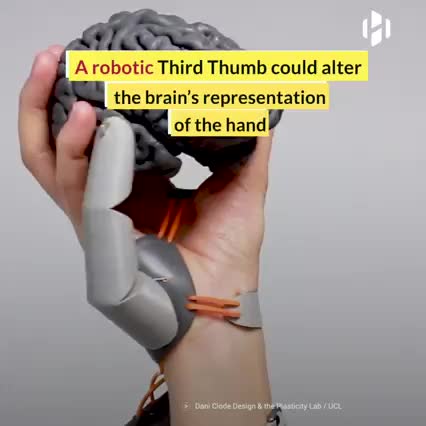

Credit: Dani Clode. #Neuroscience #Research #Biology #Technology

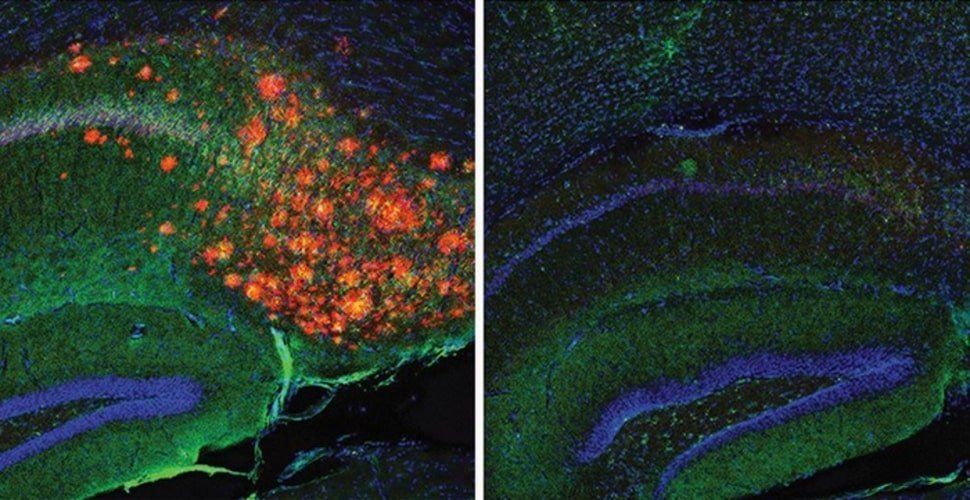
“We showed that cholesterol is acting essentially as a signal in neurons that determines how much Aβ gets made—and thus it should be unsurprising that apoE, which carries the cholesterol to neurons, influences Alzheimer’s risk,” says study co-senior author Scott Hansen, PhD, an associate professor in the Department of Molecular Medicine at Scripps Research, Florida.
Summary: A new advanced imaging technique shows how cholesterol regulates the production of Alzheimer’s associated amyloid beta proteins in astrocytes.
Source: Scripps Research Institute
A team co-led by scientists at Scripps Research has used advanced imaging methods to reveal how the production of the Alzheimer’s-associated protein amyloid beta (Aβ) in the brain is tightly regulated by cholesterol.
Appearing on line Thursday ahead of print in the Aug. 17 issue of the Proceedings of the National Academy of Sciences (PNAS), the scientists’ work advances understanding of how Alzheimer’s disease develops and underscores the long-underappreciated role of brain cholesterol.
This is interesting. 😃
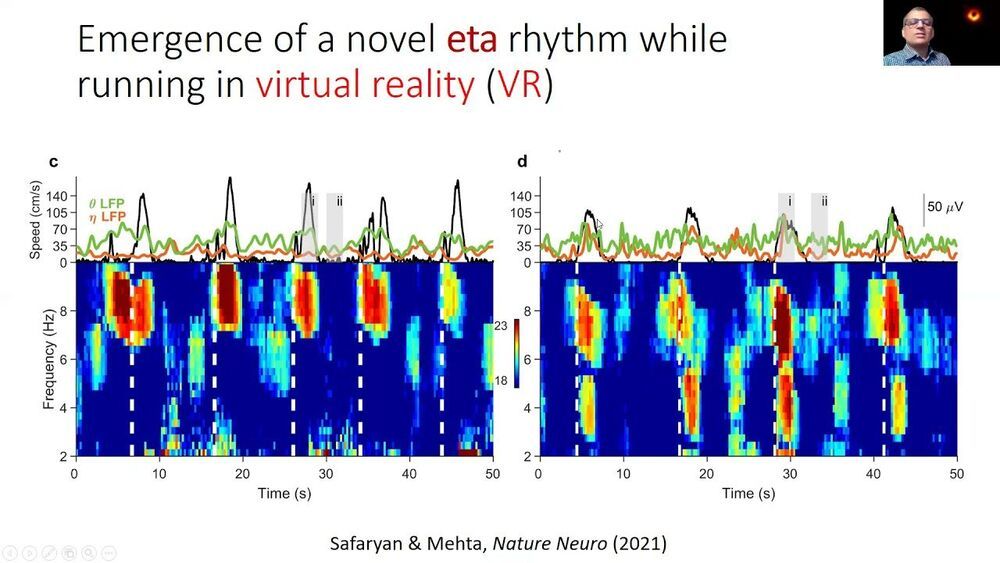
A new discovery in rats shows that the brain responds differently in immersive virtual reality environments versus the real world. The finding could help scientists understand how the brain brings together sensory information from different sources to create a cohesive picture of the world around us. It could also pave the way for “virtual reality therapy” for learning and memory-related disorders ranging including ADHD, Autism, Alzheimer’s disease, epilepsy and depression.
Mayank Mehta, PhD, is the head of W. M. Keck Center for Neurophysics and a professor in the departments of physics, neurology, and electrical and computer engineering at UCLA. His laboratory studies a brain region called the hippocampus, which is a primary driver of learning and memory, including spatial navigation. To understand its role in learning and memory, the hippocampus has been extensively studied in rats as they perform spatial navigation tasks.
When rats walk around, neurons in this part of the brain synchronize their electrical activity at a rate of 8 pulses per second, or 8 Hz. This is a type of brain wave known as the “theta rhythm,” and it was discovered more than six decades ago.
Eight months in, 2,021 has already become a record year in brain-computer interface (BCI) funding, tripling the $97 million raised in 2019.

“Our research may help us understand how abnormalities in anxiety-like behavior occur and design circuit-based therapeutic approaches for correcting them,” remarks Professor Ji Won Um from the Department of Brain and Cognitive Sciences at DGIST, who led the study.
Summary: Study identifies the role a specific protein plays in regulating the development of inhibitory synapses in the hippocampus in the context of anxiety-related behaviors.
Source: DGIST
The mechanisms behind the organization of neuronal synapses remain unclear owing to the sheer number of genes, proteins, and neuron types involved. In a recent study, Daegu Gyeongbuk Institute of Science and Technology scientists conducted a series of experiments in genetically modified mice to clarify the role of two proteins in regulating the development of inhibitory synapses in the hippocampus, in the context of anxiety-related behaviors, paving the way to better understand the brain.
The correct functioning of our brain, as well as that of other animals, depends on a very intricate interplay between multiple types of neurons. These interactions are orchestrated by multitudes of synaptic proteins; thus, pinpointing their specific functions is extremely challenging. In particular, the molecular mechanisms that regulate the plasticity of synapses are not completely understood.
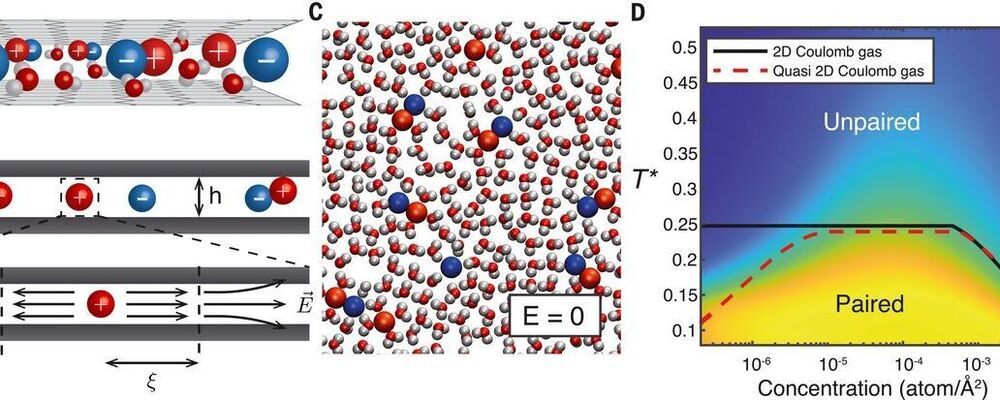
Most memory resistor (“memristor”) systems use electrons as the charge carrier, but it may also be possible to use ionic carriers, similar to the way that neurons work. Robin et al. studied an aqueous electrolyte confined into a pseudo two-dimensional gap between two graphite layers (see the Perspective by Hou and Hou). The authors observed a current–voltage relation that exhibits hysteresis, and the conductance depends on the history of the system, also known as the memresistor effect. Using simulations of their system, they can model the emission of voltage spikes characteristic of neuromorphic activity.
Science, abf7923, this issue p. 687; see also abj0437, p. 628
Recent advances in nanofluidics have enabled the confinement of water down to a single molecular layer. Such monolayer electrolytes show promise in achieving bioinspired functionalities through molecular control of ion transport. However, the understanding of ion dynamics in these systems is still scarce. Here, we develop an analytical theory, backed up by molecular dynamics simulations, that predicts strongly nonlinear effects in ion transport across quasi–two-dimensional slits. We show that under an electric field, ions assemble into elongated clusters, whose slow dynamics result in hysteretic conduction. This phenomenon, known as the memristor effect, can be harnessed to build an elementary neuron. As a proof of concept, we carry out molecular simulations of two nanofluidic slits that reproduce the Hodgkin-Huxley model and observe spontaneous emission of voltage spikes characteristic of neuromorphic activity.

