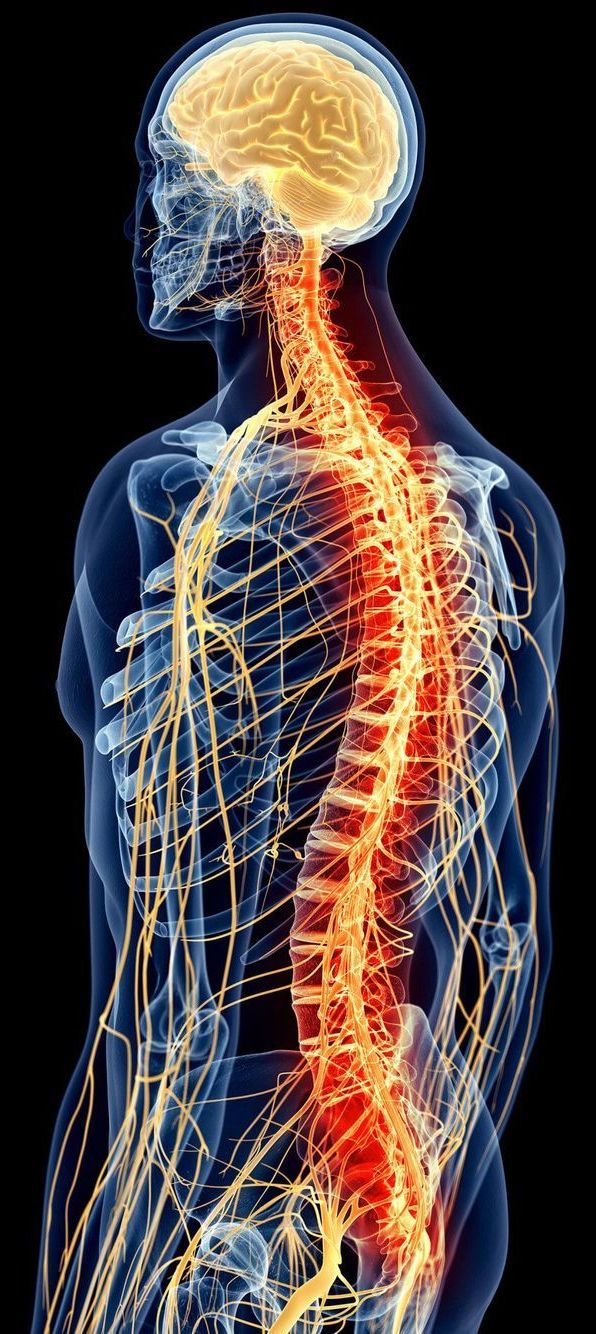
The information-processing capabilities of the brain are often reported to reside in the trillions of connections that wire its neurons together. But over the past few decades, mounting research has quietly shifted some of the attention to individual neurons, which seem to shoulder much more computational responsibility than once seemed imaginable.
The latest in a long line of evidence comes from scientists’ discovery of a new type of electrical signal in the upper layers of the human cortex. Laboratory and modeling studies have already shown that tiny compartments in the dendritic arms of cortical neurons can each perform complicated operations in mathematical logic. But now it seems that individual dendritic compartments can also perform a particular computation — “exclusive OR” — that mathematical theorists had previously categorized as unsolvable by single-neuron systems.
“I believe that we’re just scratching the surface of what these neurons are really doing,” said Albert Gidon, a postdoctoral fellow at Humboldt University of Berlin and the first author of the paper that presented these findings in Science earlier this month.
The discovery marks a growing need for studies of the nervous system to consider the implications of individual neurons as extensive information processors. “Brains may be far more complicated than we think,” said Konrad Kording, a computational neuroscientist at the University of Pennsylvania, who did not participate in the recent work. It may also prompt some computer scientists to reappraise strategies for artificial neural networks, which have traditionally been built based on a view of neurons as simple, unintelligent switches.
The Limitations of Dumb Neurons
In the 1940s and ’50s, a picture began to dominate neuroscience: that of the “dumb” neuron, a simple integrator, a point in a network that merely summed up its inputs. Branched extensions of the cell, called dendrites, would receive thousands of signals from neighboring neurons — some excitatory, some inhibitory. In the body of the neuron, all those signals would be weighted and tallied, and if the total exceeded some threshold, the neuron fired a series of electrical pulses (action potentials) that directed the stimulation of adjacent neurons.
At around the same time, researchers realized that a single neuron could also function as a logic gate, akin to those in digital circuits (although it still isn’t clear how much the brain really computes this way when processing information). A neuron was effectively an AND gate, for instance, if it fired only after receiving some sufficient number of inputs.
The dendritic arms of some human neurons can perform logic operations that once seemed to require whole neural networks.