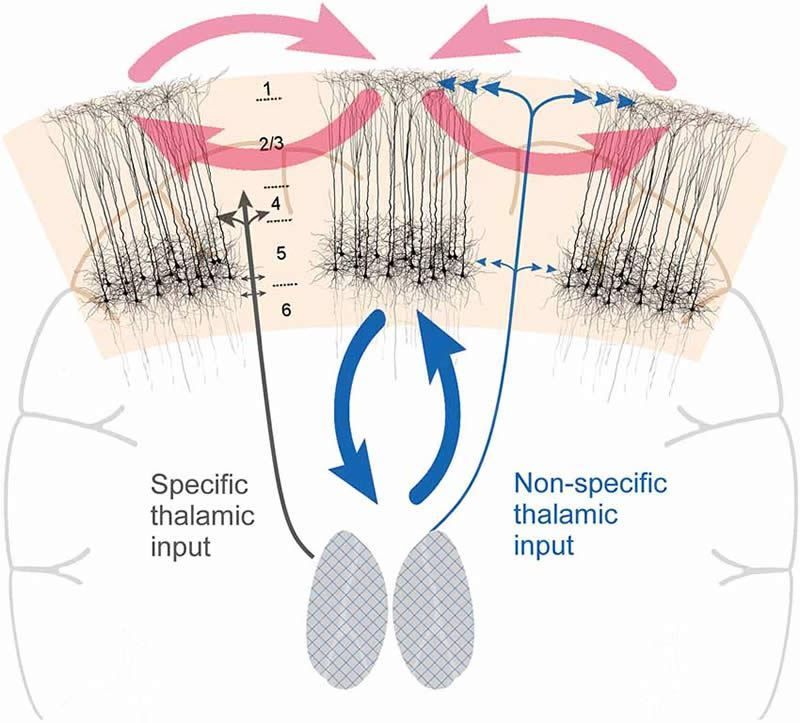
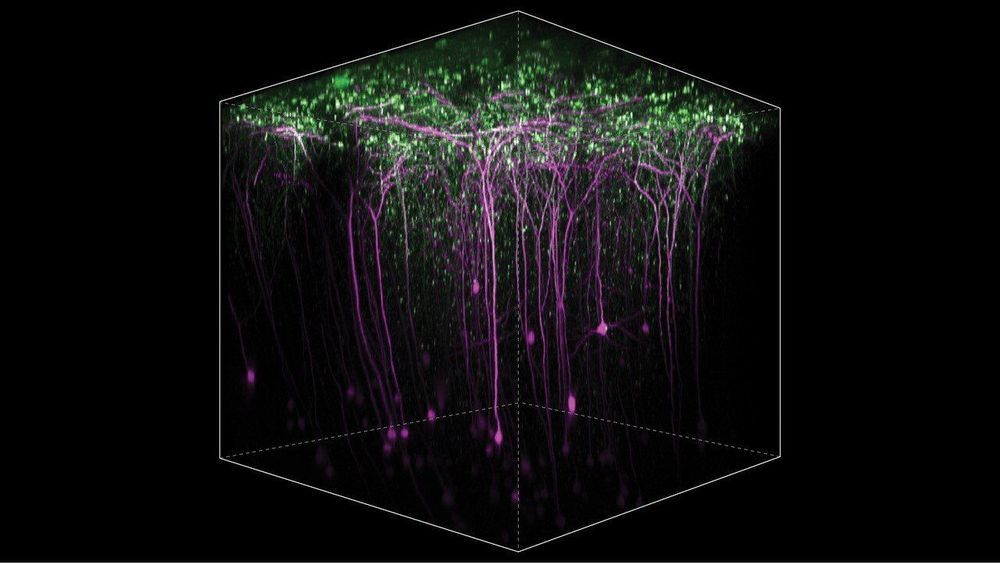
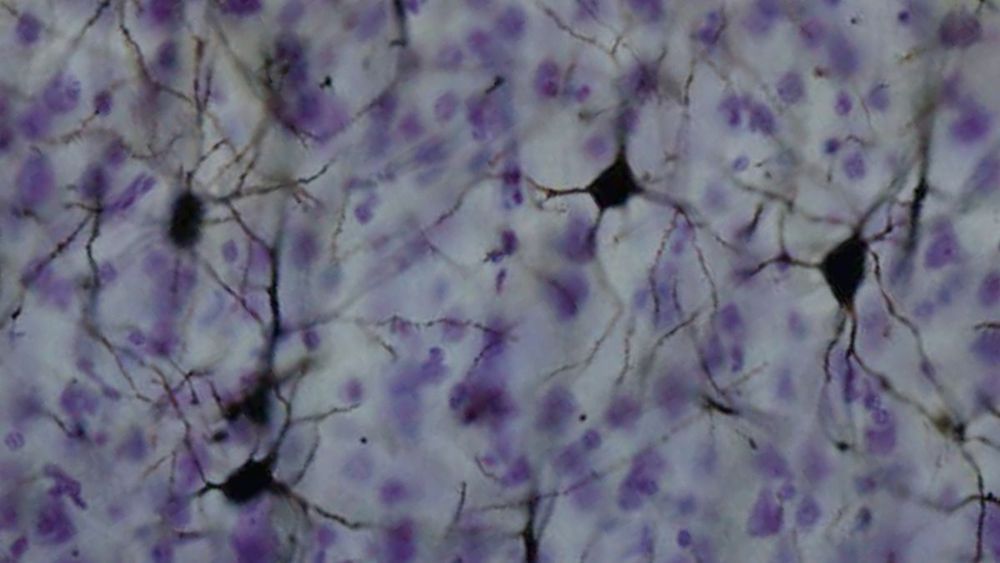
Neurological conditions or injuries that result in the inability to communicate can be devastating. Patients with such speech loss often rely on alternative communication devices that use brain–computer interfaces (BCIs) or nonverbal head or eye movements to control a cursor to spell out words. While these systems can enhance quality-of-life, they can only produce around 5–10 words per minute, far slower than the natural rate of human speech.
Researchers from the University of California San Francisco today published details of a neural decoder that can transform brain activity into intelligible synthesized speech at the rate of a fluent speaker (Nature 10.1038/s41586-019‑1119-1).
“It has been a longstanding goal of our lab to create technology to restore communication for patients with severe speech disabilities,” explains neurosurgeon Edward Chang. “We want to create technologies that can generate synthesized speech directly from human brain activity. This study provides a proof-of-principle that this is possible.”