A years-long Allen Institute for Brain Science project to map the entire mouse brain is complete, and you can explore it now.
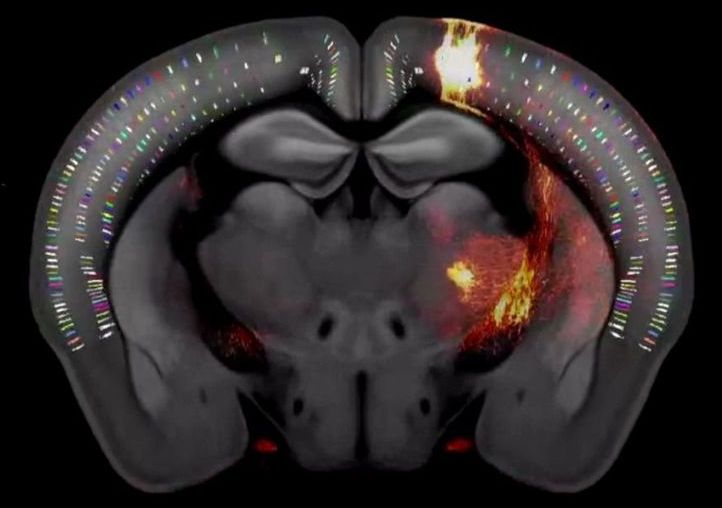

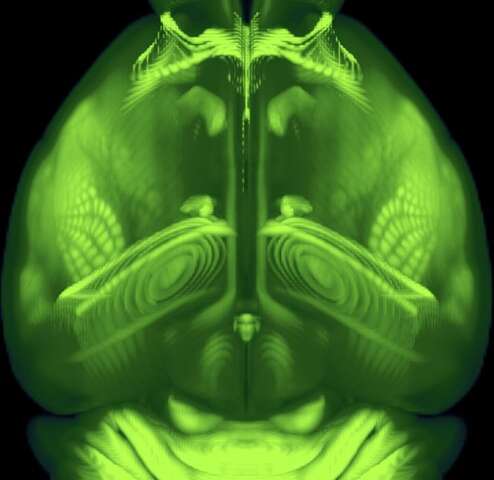
After three years of intensive data-gathering and careful drawing, the mapmakers’ work was complete.
The complex terrain they charted, with all its peaks, valleys and borders, is only about half an inch long and weighs less than a jellybean: the brain of the laboratory mouse.
In a paper published today in the journal Cell, the Allen Institute mapmakers describe this cartographical feat—the third iteration of the Allen Mouse Brain Common Coordinate Framework, or CCFv3 (https://portal.brain-map.org/), a complete, high-resolution 3D atlas of the mouse brain.

Recent sleep surveys show that Singaporeans are among the world’s most sleep-deprived people. Scientists from Duke-NUS Medical School (Duke-NUS) and the University of Tokyo provide new evidence, which supports the presence of a key mechanism that regulates our biological clock. In the study published in PNAS, the team used preclinical models to validate that mutations in PER2 protein can alter the balance of the circadian period, which can lead to sleep disorders.
Biological clocks are an organism’s innate timing device. It is composed of specific proteins called clock proteins, which interact in cells throughout the body. Biological clocks produce and regulate circadian rhythms —the physical, mental, and behavioral changes that follow a daily cycle. Understanding the molecular mechanisms of the circadian clock provides a huge potential to identify therapeutic interventions to mitigate circadian disruption, and its long-term consequences such as diabetes, obesity and cancer among shift workers, who undergo frequent circadian disruption and are more prone to these diseases.
The Duke-NUS scientists had previously discovered that mutations in a specific protein (called casein kinase 1) alters the core clock protein (called PERIOD or PER), and this changes the timing of the biological clock. In this study, preclinical models were used to investigate the role of PER2 (a type of PER protein) in clock regulation to further understand and strengthen the model.

https://www.facebook.com/383136302314720/posts/578325486129133/
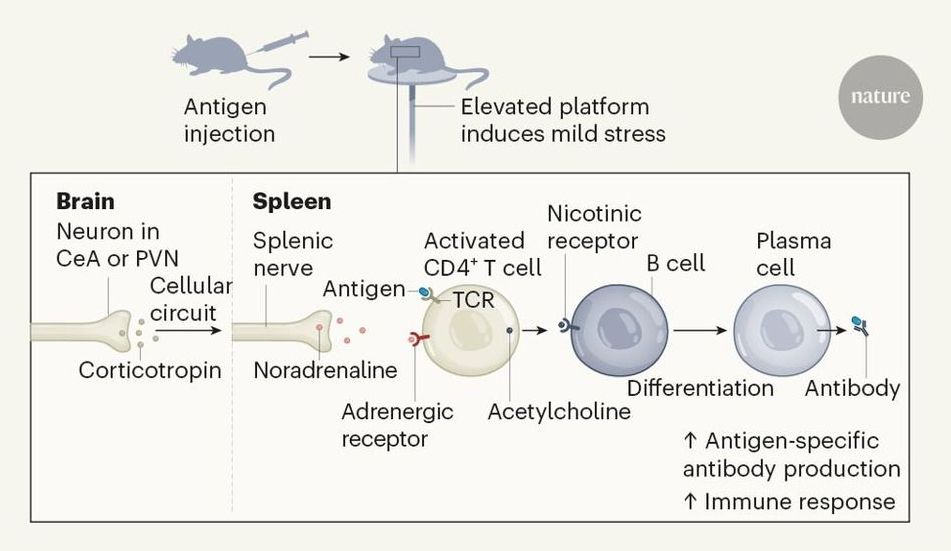
Elucidating how the brain controls peripheral organs in the fight against infection is crucial for our understanding of brain–body interactions. A study in mice reveals one such pathway worthy of further investigation. Neurons in stress-responsive brain regions boost plasma-cell formation.

A study just released by Columbia University Mailman School of Public Health is reporting a blood-DNA-methylation measure that is sensitive to variation in the pace of biological aging among individuals born the same year. The tool—DunedinPoAm—offers a unique measurement for intervention trials and natural experiment studies investigating how the rate of aging may be changed by behavioral or drug therapy, or by changes to the environment. The study findings are published online in the journal eLife.
“The goal of our study was to distill a measurement of the rate of biological aging based on 12-years of follow-up on 18 different clinical tests into a blood test that can be administered at a single time point.” said lead author Daniel Belsky, Ph.D., assistant professor of epidemiology at Columbia Mailman School and a researcher at the Columbia Aging Center.
Midlife adults measured to be aging faster according to the new measurement showed faster declines in physical and cognitive functioning and looked older in facial photographs. Older adults measured to be aging faster by the tool were at increased risk for chronic disease and mortality. In other analyses, the researchers showed that DunedinPoAm captured new information not measured by proposed measures of biological aging known as epigenetic clocks, that 18-year-olds with histories of childhood poverty and victimization showed faster aging as measured by DunedinPoAm, and that DunedinPoAm predictions were disrupted by a caloric restriction intervention in a randomized trial.

World-first research led by neuroimaging expert Dr. Jiyang Jiang at UNSW’s Centre for Healthy Brain Ageing (CHeBA) has found that those aged 95 and over demonstrated more activation between the left and ride side of their brain than their younger counterparts.
Given the prevalence of dementia increases with age, near-centenarians and centenarians without dementia are generally considered as models of successful aging and resistance against age-related cognitive decline.
“We wanted to see if there was something particularly special about the brain’s functional connectivity of those aged 95 and older that helps them preserve brain function into the 11th decade of their life,” says Dr. Jiang.

Fyodor Rouge, this is the church I was telling you about.
The Bradenton-based organization falsely claims that the treatment is effective for a number of conditions, including Alzheimer’s disease, brain disease, cancer, HIV and AIDS, according to the Food and Drug Administration.
This content is being provided for free as a public service to our readers during the coronavirus outbreak. Sign up for our daily or breaking newsletters to stay informed. If local news is important to you, consider becoming a digital subscriber to the Sarasota Herald-Tribune.
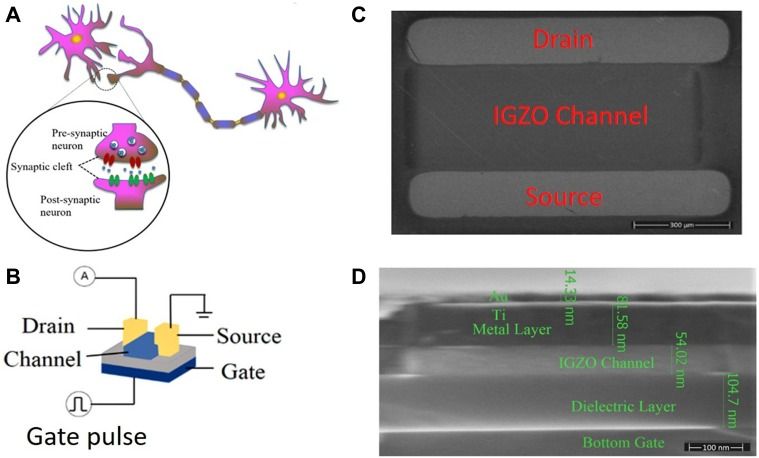
Electronic devices which mimic the functionality of biological synapses are a large step to replicate the human brain for neuromorphic computing and for numerous medical research investigations. One of the representative synaptic behaviors is paired-pulse facilitation (PPF). It has been widely investigated because it is regarded to be related to biological memory. However, plasticity behavior is only part of the human brain memory behavior.
Here, we present a phenomenon which is opposite to PPF, i.e., paired-pulse inhibition (PPI), in nano oxide devices for the first time. The research here suggests that rather than being enhanced, the phenomena of memory loss would also be possessed by such electronic devices. The device physics mechanism behind memory loss behavior was investigated. This mechanism is sustained by historical memory and degradation manufactured by device trauma to regulate characteristically stimulated origins of artificial transmission behaviors.
Under the trauma of a memory device, both the signal amplitude and signal time stimulated by a pulse are lower than the first signal stimulated by a previous pulse in the PPF, representing a new scenario in the struggle for memory. In this way, more typical human brain behaviors could be simulated, including the effect of age on latency and error generation, cerebellar infarct, trauma and memory loss pharmacological actions (such as those caused by hyoscines and nitrazepam).