Circa 2019
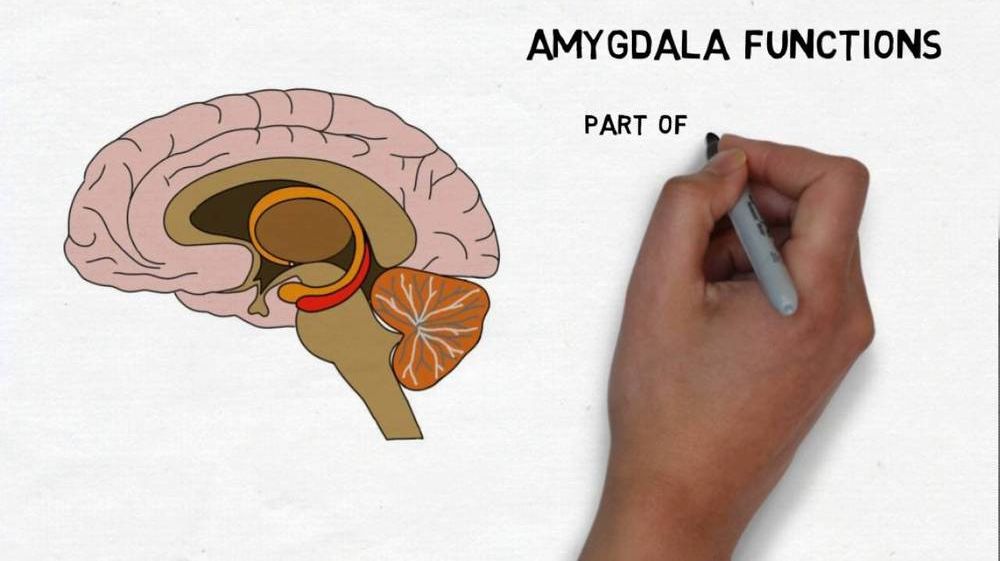
Cerebral palsy is a condition that results from injuries or abnormalities of the brain, usually in the womb but occurring any time during 2 years after birth. It affects brain and nervous system functions such as thinking, seeing, hearing, learning and movement.
Common causes are hypoxia (low oxygen levels), head injury, maternal infections such as rubella, brain bleeding, brain infection, and severe jaundice. Types of CP include: ataxic, hypotonic, spastic, dyskinetic, and mixed.
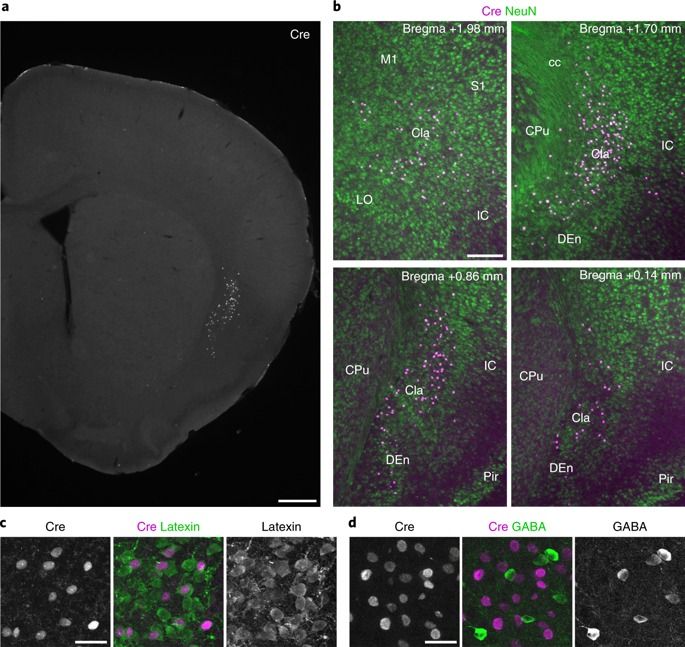
Mesenchymal stem cells from umbilical cord are considered to be universal donor cells because they are not immediately recognized as foreign. The cells home to damaged tissue and are known to secrete molecules called trophic factors.