#DigitalTheology #TheologyofDigitalPhysics #PhenomenalConsciousness #CosmicSelf #HolographicPrinciple #DigitalPhysics #theology #pantheism #consciousness
Since we live in a world which isn’t random, but organized at every level, a role for consciousness seems unavoidable. The ‘digital theologian’ shows us compelling evidence from quantum mechanics, mathematics and computer sciences, which not only aligns with a philosophical worldview of the Primacy of Consciousness, but which also assigns a role to information as its modus operandi.
It is quantum mechanics which appears to connect the Universe as a whole to consciousness. A whole, which is more than the sum of its parts and irreducible to mere assumptions deriving from the anatomizing dissection into mental confabulations. Drawing from the holographic principle, perceptroniums and noocentrism, Alex provides crucial keys to unlock the mystery of consciousness to show us how our local consciousness can arise from a non-local cosmic consciousness network.
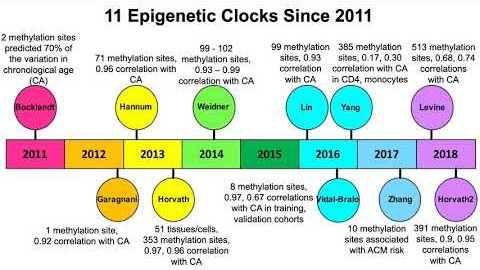
Carefully building his fortress of arguments, Alex gathers his building bricks from various areas of scientific exploration, ranging from the role of language and tools in the development of our consciousness, the physics of time and epigenetics. Traditional Darwinism and reductive materialism become so challenged, that we become bound to agree with Terence McKenna’s statement that “object fetishism is completely bankrupt.” All these threads are then skillfully woven into the irresistible attractor and only logical conclusion, or Digital Pantheism and Omega Point Cosmology. And with this thus synthesized Apotheosis, Vikoulov brings the architecture of his chef-d’oeuvre to full fruition.