Her birth represents the first cloning of an endangered species native to North America, and may bring needed genetic diversity to the species.



This is the FIRST part of the interview with Rodolfo Goya.
In this video Professor Goya talks about his role in the original experiment and the progress in his current study to reproduce the results with young blood plasma.
Professor Rodolfo Goya is Senior Scientist at The National Scientific and Technical Research Council in Argentina where he is a biochemist and researcher.
Dr. Goya has led a number of studies on cellular reprogramming and restoration of function in important organs, such as the thymus and the brain. He is also studying different aspects of cryopreservation.
He was one of the authors of the paper “Reversing age: dual species measurement of epigenetic age with a single clock” and is now working in his lab to reproduce and extend the results.
If you would like to support our channel, we’d love a coffee…thank you!
https://www.buymeacoffee.com/mhealthspan

Almost one in five people lack the protein α-aktinin-3 in their muscle fiber. Researchers at Karolinska Institutet in Sweden now show that more of the skeletal muscle of these individuals comprises slow-twitch muscle fibers, which are more durable and energy-efficient and provide better tolerance to low temperatures than fast-twitch muscle fibers. The results are published in the scientific journal The American Journal of Human Genetics.
Skeletal muscle comprises fast-twitch (white) fibers that fatigue quickly and slow-twitch (red) fibers that are more resistant to fatigue. The protein α-aktinin-3, which is found only in fast-twitch fibers, is absent in almost 20 percent of people – almost 1.5 billion individuals – due to a mutation in the gene that codes for it. In evolutionary terms, the presence of the mutated gene increased when humans migrated from Africa to the colder climates of central and northern Europe.
“This suggests that people lacking α-aktinin-3 are better at keeping warm and, energy-wise, at enduring a tougher climate, but there hasn’t been any direct experimental evidence for this before,” says Håkan Westerblad, professor of cellular muscle physiology at the Department of Physiology and Pharmacology, Karolinska Institutet. “We can now show that the loss of this protein gives a greater resilience to cold and we’ve also found a possible mechanism for this.”

While the brain tissue of modern humans are typically smooth and spherical, the study, which was published in Science on Feb 11, found that the tissue created with the ancient genes were smaller and had rough, complex surfaces.
“The question here is what makes us human,” Muotri told CNN. “Why are our brains so different from other species including our own extinct relatives?”

An international team led by researchers at the Centre for Palaeogenetics in Stockholm has sequenced DNA recovered from mammoth remains that are up to 1.2 million years old. The analyses show that the Columbian mammoth that inhabited North America during the last ice age was a hybrid between the woolly mammoth and a previously unknown genetic lineage of mammoth. In addition, the study provides new insights into when and how fast mammoths became adapted to cold climate. These findings are published today (February 172021) in Nature.
Around one million years ago there were no woolly or Columbian mammoths, as they had not yet evolved. This was the time of their predecessor, the ancient steppe mammoth. Researchers have now managed to analyze the genomes from three ancient mammoths, using DNA recovered from mammoth teeth that had been buried for 0.7−1.2 million years in the Siberian permafrost.
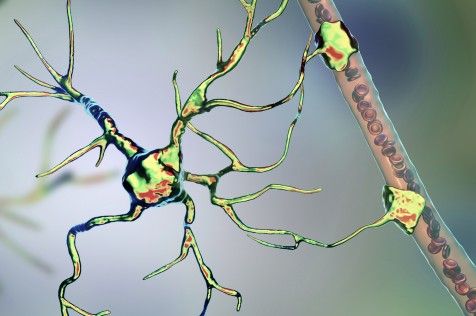
The human genome contains billions of pieces of information and around 22000 genes, but not all of it is, strictly speaking, human. Eight percent of our DNA consists of remnants of ancient viruses, and another 40 percent is made up of repetitive strings of genetic letters that is also thought to have a viral origin. Those extensive viral regions are much more than evolutionary relics: They may be deeply involved with a wide range of diseases including multiple sclerosis, hemophilia, and amyotrophic lateral sclerosis (ALS), along with certain types of dementia and cancer.
For many years, biologists had little understanding of how that connection worked—so little that they came to refer to the viral part of our DNA as dark matter within the genome. “They just meant they didn’t know what it was or what it did,” explains Molly Gale Hammell, an associate professor at Cold Spring Harbor Laboratory. It became evident that the virus-related sections of the genetic code do not participate in the normal construction and regulation of the body. But in that case, how do they contribute to disease?
Eight percent of our DNA consists of remnants of ancient viruses, and another 40 percent is made up of repetitive strings of genetic letters that is also thought to have a viral origin.
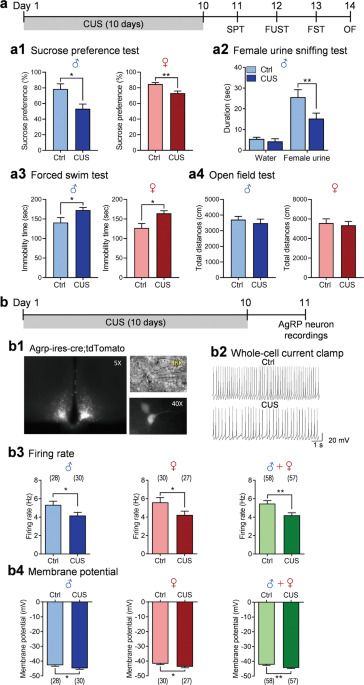
Previous studies have shown that AgRP neurons in the arcuate nucleus (ARC) respond to energy deficits and play a key role in the control of feeding behavior and metabolism. Here, we demonstrate that chronic unpredictable stress, an animal model of depression, decreases spontaneous firing rates, increases firing irregularity and alters the firing properties of AgRP neurons in both male and female mice. These changes are associated with enhanced inhibitory synaptic transmission and reduced intrinsic neuronal excitability. Chemogenetic inhibition of AgRP neurons increases susceptibility to subthreshold unpredictable stress. Conversely, chemogenetic activation of AgRP neurons completely reverses anhedonic and despair behaviors induced by chronic unpredictable stress.

The adeno-associated viruses often used as vectors to deliver gene therapy can trigger unwanted and sometimes dangerous immune responses. Now, a Harvard University team led by renowned geneticist George Church, Ph.D., has developed a way to “cloak” AAVs from immune surveillance. They’ve spun off Ally Therapeutics to develop it.
After tracing the origins of schizophrenia to genes expressed in the placenta while in utero, scientists have now zeroed in on the combination of risk factors that could predict which infants are at greatest risk of developing the condition later in life.
The findings reinforce an emerging picture of schizophrenia as a genetic disorder, with a fate determined by complications that can arise during pregnancy.
Researchers from the Lieber Institute for Brain Development at Johns Hopkins University and the University of North Carolina in the US analysed the relationship between key genes and cognitive development in the first few years after birth.
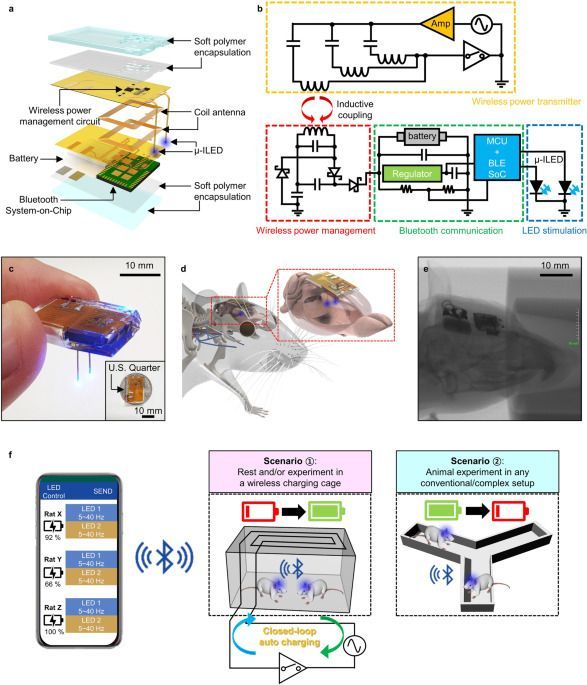
Although wireless optogenetic technologies enable brain circuit investigation in freely moving animals, existing devices have limited their full potential, requiring special power setups. Here, the authors report fully implantable optogenetic systems that allow intervention-free wireless charging and controls for operation in any environment.