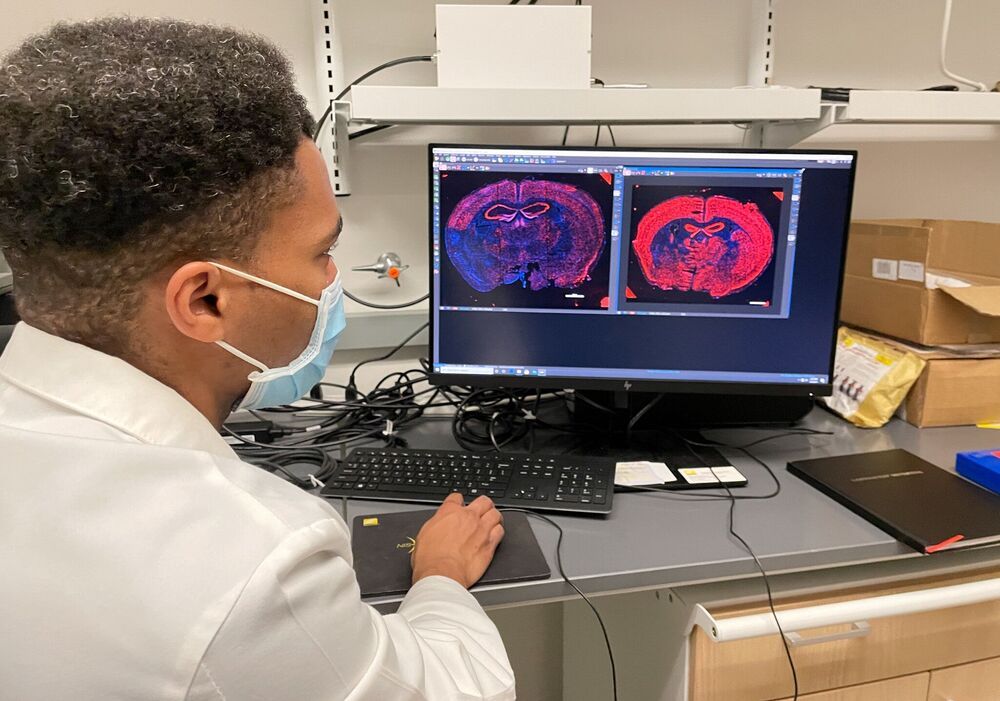
Long but annotated! Most important here is human data for specific treatments due out starting in May or June. And apparently they had a mouse study where they expected a paper due out already but other groups chimed in to help with more testing so there is a delay.
Liz Parrish, CEO of BioViva Science and patient zero of biological rejuvenation with gene therapies, is interviewed by Zora Benhamou on her fresh podcast “HackMyAge”.
During the conversation, Liz enters deep into the world of gene therapies, either to cure monogenic diseases, multifactorial genetic diseases, or the mother of all diseases: aging itself.
The conversation lasts for one hour and twenty minutes and has no waste. However, to go direct to certain themes use the following time marks:
0:00:00 Zora introduces the podcast: who is Liz Parrish and what the conversation will be about.
0:02:17 Liz Parrish begins her intervention in the podcast.
0:03:00 What is gene therapy and how Liz got involved in gene therapy applied to aging.
0:05:52 How Liz and her son are dealing with the treatment of type 1 diabetes.
0:08:05 How Liz got involved in becoming the first human treated with gene therapy to treat biological aging and what it means to go through gene therapy.
0:14:34 Current legal status of gene therapies and ways to get the treatment.
0:16:20 Current costs of undergoing gene therapies.
0:18:49 Why aren’t medical doctors applying gene therapies more than they actually are and what’s the role of medical tourism.
0:21:34 What prompted Liz to become the first patient to undergo gene therapy for biological aging.
0:25:25 How gene therapies compare with nutraceuticals and pharmaceuticals.
0:30:05 Why big pharmaceutical companies haven’t jumped into the field with more impetus.
0:33:20 How long will it take for gene therapies to become mainstream.
0:39:29 How gene therapies work and what is the experience for the patient that goes through it.
0:48:11 What can be expected from treating sarcopenia with gene therapy.
0:50:02 Where do the genes used in gene therapies come from.
0:53:12 What can expect someone who is treated with gene therapy to tackle dementia.
0:54:34 What are the major changes experienced by Liz in her blood markers since being treated.
0:56:38 When and how did Liz go through her gene therapies, not only for hTERT and Follistatin but also for Klotho and PGC-1alpha. What are the latter two all about?
1:02:15 How Liz envision the future of humans.
1:04:08 Liz comments on a coming paper BioViva is working together with Rutgers University.
1:05:38 Other studies in the pipeline.
1:06:45 Explanation of testing services and data storage offered by BioViva.
1:17:20 Liz on topical creams and/or small molecules for removal of senescent cells, and pills for telomeres lengthening.
1:19:16 Liz responds to “if you could meet your 20-year-old self what would you tell her”
1:20:03 What can people do to help Liz on her mission.
1:22:12 Resources to learn more about the future that is coming, genomics and gene therapy technology.
1:24:18 BioViva and Integrated Health Systems websites as well as social media sites where Liz and BioViva are actively present.
1:25:38 Words of farewell.
Websites:
HackMyAge: https://hackmyage.com/
BioViva Science: https://bioviva-science.com/
Integrated Health Systems: https://www.integrated-health-systems.com/