Ogba Educational Clinic
A nightmarish scene was burnt into my memory nearly two decades ago: Changainjie, Beijing’s normally chaotic “fifth avenue,” desolate without a sign of life. Schools shut, subways empty, people terrified to leave their homes. Every night the state TV channels reported new cases and new deaths. All the while, we had to face a chilling truth: the coronavirus, SARS, was so novel that no one understood how it spread or how to effectively treat it. No vaccines were in sight. In the end, it killed nearly 1,000 people.
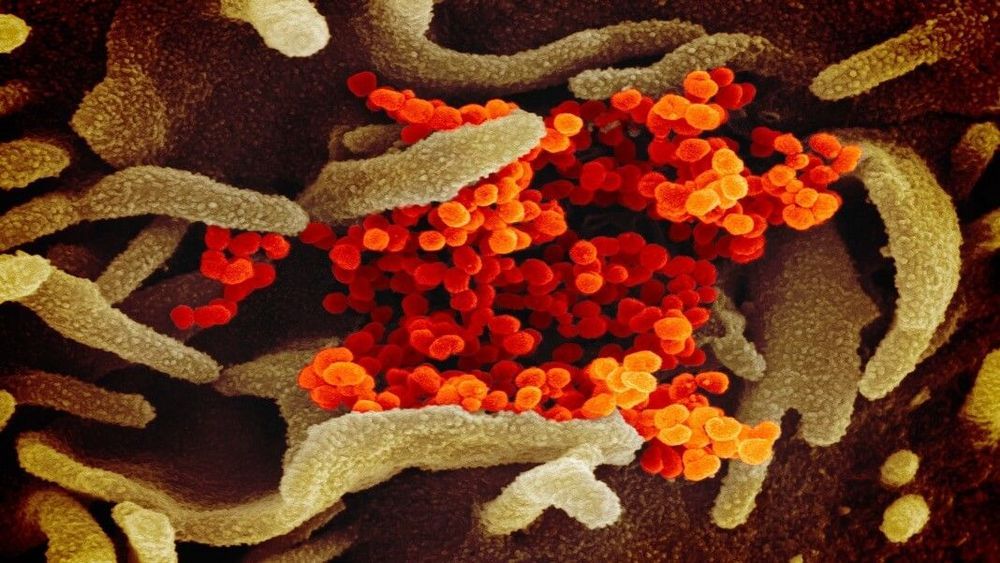
It’s impossible not to draw parallels between SARS and the new coronavirus outbreak, COVID-19, that’s been ravaging China and spreading globally. Yet the response to the two epidemics also starkly highlights how far biotech and global collaborations have evolved in the past two decades. Advances in genetic sequencing technologies, synthetic biology, and open science are reshaping how we deal with potential global pandemics. In a way, the two epidemics hold up a mirror to science itself, reflecting both technological progress and a shift in ethos towards collaboration.
Let me be clear: any response to a new infectious disease is a murky mix of science, politics, racism, misinformation, and national egos. It’s naïve to point to better viral control and say it’s because of technology alone. Nevertheless, a comparison of the two outbreaks dramatically highlights how the scientific world has changed, for the better, in the last two decades.