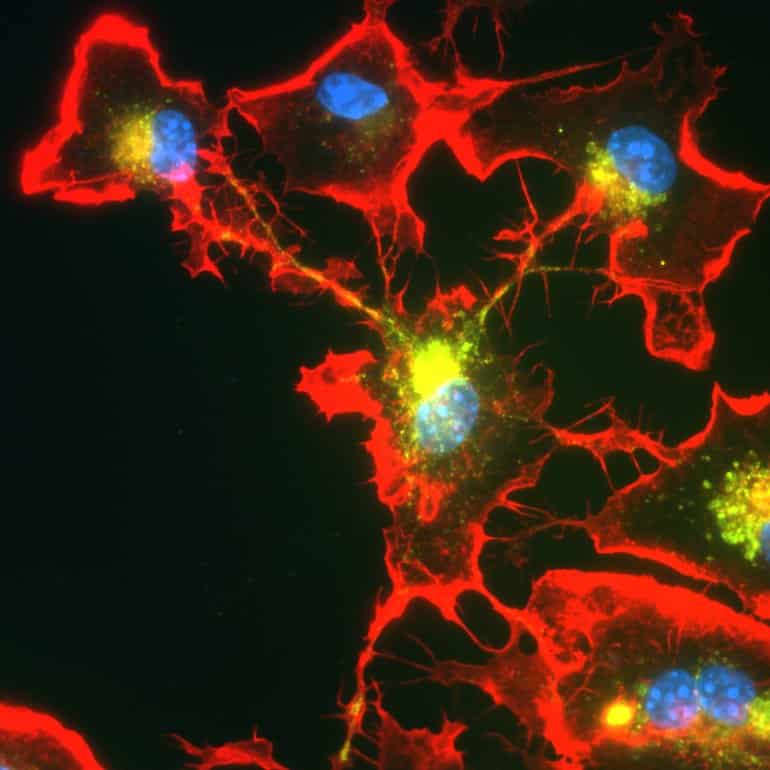
Microglia immune cells can join together to form networks when needed, a new study reports. However, certain mutations associated with Parkinson’s disease can impair this process.


Is an academic doctor and medical technology entrepreneur, working in the field of the computational biology of aging.
Dr. Radenkovic is also a Partner at the SALT Bio-Fund, and a co-founder of Hooke, an elite longevity research clinic in London.
Dr. Radenkovic has a dual degree in medicine and physiology from University College London Medical School, and did her residency at St Thomas’ Hospital in London. She later worked as Research Fellow at King’s College London and at Harvard University.
Dr. Radenkovic has led a variety of projects, including a digital therapeutics company for women and an algorithm for cardiac MRI based on fractal geometry, to major industry acquisitions.
Dr. Radenkovic has over 30 academic papers, 7 grants, and over 40 scientific conference presentations. She is fluent in 5 languages and 3 programming languages.

Bacteria may get a bad reputation in general, yet it’s actually generally healthy and serves an important role in many habitats, including human bodies. From supporting life on Earth to being employed in industrial and medicinal processes, bacteria have their figurative fingers in many pots — some varieties of bacteria can even filter tainted water and make it safe for human consumption.
A team of researchers from the Indian Institute of Technology, Banaras Hindu University (IIT-BHU) has found a bacteria that can do just that — Named “microbacterium paraoxydans strain VSVM IIT (BHU)” by the scientists, it can separate toxic hexavalent chromium from water in an effective and eco-friendly manner, according to a research published in the Journal of Environmental Chemical Engineering.
Hexavalent chromium is a heavy metal ion that is used in electroplating, welding, and chromate painting, among other things. It’s said to be responsible for health problems in humans like cancers, kidney and liver malfunctioning, and infertility. When compared to current approaches, the scientists believe that this bacterial strain, which can tolerate high amounts of hexavalent chromium, is particularly successful at eliminating the harmful substance from wastewater.

Advocates of the fat-but-fit approach to obesity treatment argue that improving fitness, even in the absence of weight loss, can help reduce the risk of cardiovascular disease and mortality.
New research suggests that improving fitness is at least as effective as weight loss for staving off obesity-related cardiovascular disease and mortality risks.
From ecosystem development to talent, much effort is still required for practical implementation of edge AI.
By Pushkar Apte and Tom Salmon
Rapid advances in artificial intelligence (AI) have made this technology important for many industries, including finance, energy, healthcare, and microelectronics. AI is driving a multi-trillion-dollar global market while helping to solve some tough societal problems such as tracking the current pandemic and predicting the severity of climate-driven events like hurricanes and wildfires.
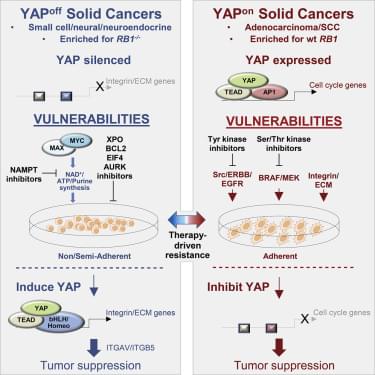
Cancer heterogeneity impacts therapeutic response, driving efforts to discover over-arching rules that supersede variability. Here, we define pan-cancer binary classes based on distinct expression of YAP and YAP-responsive adhesion regulators. Combining informatics with in vivo and in vitro gain-and loss-of-function studies across multiple murine and human tumor types, we show that opposite pro-or anti-cancer YAP activity functionally defines binary YAPon or YAPoff cancer classes that express or silence YAP, respectively. YAPoff solid cancers are neural/neuroendocrine and frequently RB1−/−, such as retinoblastoma, small cell lung cancer, and neuroendocrine prostate cancer. YAP silencing is intrinsic to the cell of origin, or acquired with lineage switching and drug resistance. The binary cancer groups exhibit distinct YAP-dependent adhesive behavior and pharmaceutical vulnerabilities, underscoring clinical relevance. Mechanistically, distinct YAP/TEAD enhancers in YAPoff or YAPon cancers deploy anti-cancer integrin or pro-cancer proliferative programs, respectively. YAP is thus pivotal across cancer, but in opposite ways, with therapeutic implications.
Pearson et al. demonstrate that YAP/TAZ, well-known oncogenes, are tumor suppressors in a large group of cancers. Pan-cancer analyses reveal that opposite YAP/TAZ expression, adhesive behavior, and oncogenic versus tumor suppressor YAP/TAZ activity functionally stratify binary cancer classes, which interchange to drive drug resistance. Contrasting YAPoff/YAPon classes exhibit unique vulnerabilities, facilitating therapeutic selection.

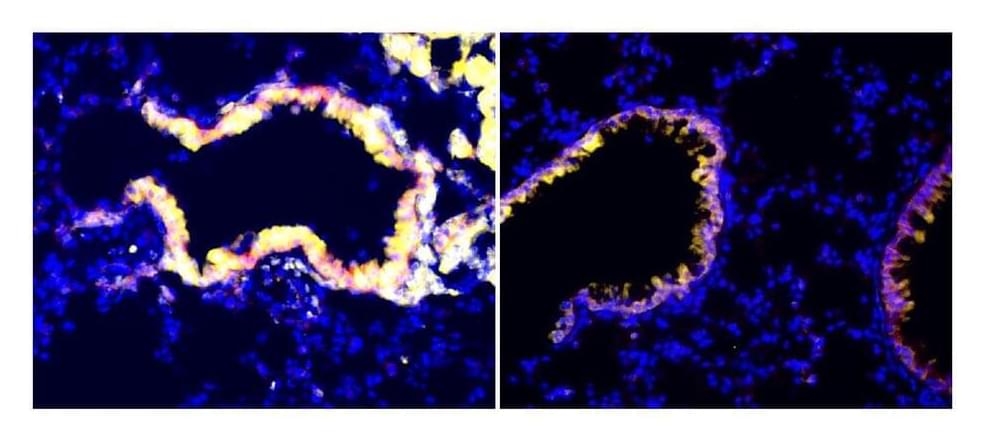
These club cell-secreted factors are able to nullify immune suppressor cells that otherwise help tumors escape an effective antitumor response,” said co-senior author Dr. Vivek Mittal, director of research at the Neuberger Berman Lung Cancer Center and the Ford-Isom Research Professor of Cardiothoracic Surgery at Weill Cornell Medicine. “We’re excited by the possibility of developing these club cell factors into a cancer treatment.
Malignant tumors can enhance their ability to survive and spread by suppressing antitumor immune cells in their vicinity, but a study led by researchers at Weill Cornell Medicine and NewYork-Presbyterian has uncovered a new way to counter this immunosuppressive effect.
In the study, published Sept. 20 in Nature Cancer, the researchers identified a set of anti-immunosuppressive factors that can be secreted by cells called club cells that line airways in the lungs. They showed in a mouse model of lung cancer that these club cell factors inhibit highly potent immunosuppressive cells called myeloid-derived suppressor cells (MDSCs), which tumors often recruit to help them evade antitumor immune responses.
The inhibition of the MDSCs led to an increase in the number of antitumor T cells at the tumor site, and greatly improved the effectiveness of FDA approved PD1 immunotherapy.
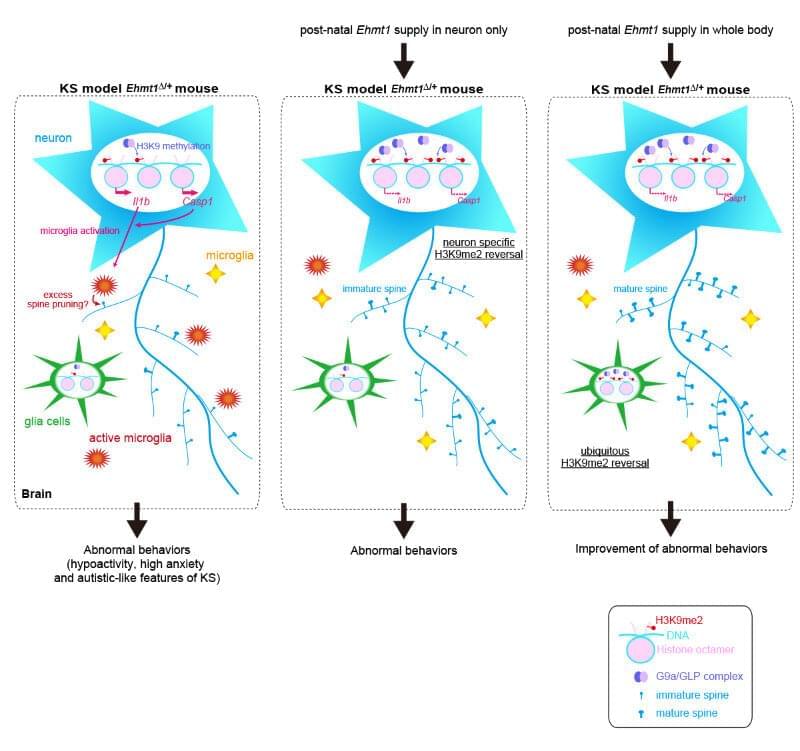
As this is the first report of neuro-inflammation in Kleefstra syndrome, the next step is to find out if it also occurs in the human condition. Shinkai believes the chances are high and says he would not be surprised if other neurological diseases caused by epigenetic dysregulation were also related to abnormal inflammation in the brain.
Researchers at the RIKEN Cluster for Pioneering Research (CPR) in Japan report that Kleefstra syndrome, a genetic disorder that leads to intellectual disability, can be reversed after birth in a mouse model of the disease. Published in the scientific journal iScience, the series of experiments led by Yoichi Shinkai showed that postnatal treatment resulted in improved symptoms, both in the brain and in behavior.
Normally, we get two good copies of most genes, one from each parent. In Kleefstra syndrome, one copy of the EHMT1 gene is mutated or missing. This leads to half the normal amount of GLP, a protein whose job is to control genes related to brain development through a process called H3K9 methylation. Without enough GLP, H3K9 methylation is also reduced, and the connections between neurons in the brain do not develop normally. The result is intellectual disability and autistic-like symptoms. “We still don’t know if Kleefstra syndrome is a curable disease after birth or how this epigenetic dysregulation leads to the neurological disorder,” says Shinkai. “Our studies in mice have provided new information about what causes the behavioral abnormalities associated with the syndrome and have shown that a cure is a real possibility in the future.”
Reasoning that extra GLP might be an effective treatment, the researchers performed a series of experiments in mice that were engineered to have only one good copy of the EHMT1 gene. The brains of these mice show characteristics of the human condition, including 40% less GLP and 30% less H3K9 methylation. The mice also display several behaviors seen in humans with Kleefstra syndrome, such as reduced locomotion and greater anxiety. After each experiment, the researchers measured these factors and compared them to normal mice to see if the treatment had been effective.
