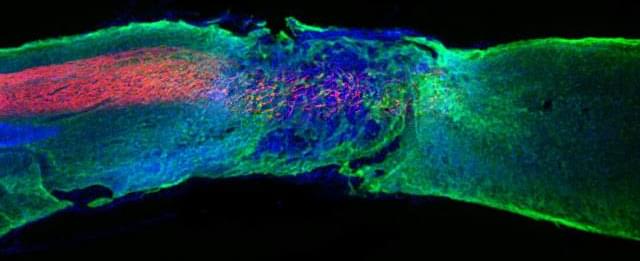
A major obstacle to widespread study and clinical use of 3D tissues is their short shelf-life, which may be anywhere from a just few hours to a few days. As in the case of an organ transplant, a bioprinted tissue must be transported rapidly to the location where it is needed, or it will not be viable. In the journal Matter on December 21st, researchers at Brigham and Women’s Hospital and Harvard Medical School describe their work combining 3D bioprinting with cryopreservative techniques to create tissues which can be preserved in a freezer at-196°C and thawed within minutes for immediate use.
“For conventional bioprinting, there is basically no shelf life. It’s really just print, and then use, in most cases,” says lead author Y. Shrike Zhang (@shrikezhang), a biomedical engineer at Brigham and Women’s Hospital. “With cryobioprinting, you can print and store in the frozen state for basically as long as you want.”
The use of 3D bioprinting to create artificial human tissue first appeared twenty years ago. As in conventional 3D printing, an ink is extruded layer by layer through a nozzle into a pre-specified shape. In the case of bioprinting, the ink is typically made up of a gelatin-like scaffolding embedded with living cells. Cryobioprinting works the same way, except the printing is performed directly onto a cold plate held at temperatures down to-20°C. After the tissues are printed, they are immediately moved to cryogenic conditions for long-term storage.