Materials scientists studying recharging fundamentals made an astonishing discovery that could open the door to better batteries, faster catalysts and other materials science leaps.
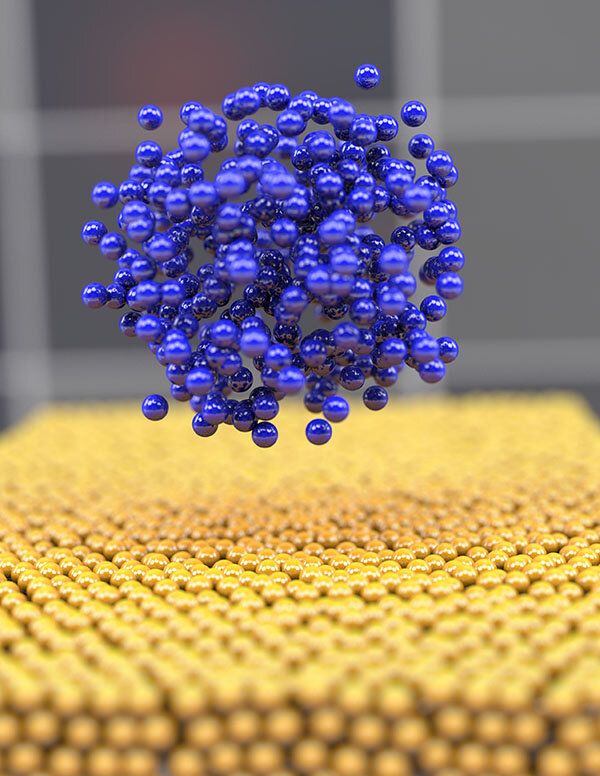
Scientists from the University of California San Diego and Idaho National Laboratory scrutinized the earliest stages of lithium recharging and learned that slow, low-energy charging causes electrodes to collect atoms in a disorganized way that improves charging behavior. This noncrystalline “glassy” lithium had never been observed, and creating such amorphous metals has traditionally been extremely difficult.
The findings suggest strategies for fine-tuning recharging approaches to boost battery life and—more intriguingly—for making glassy metals for other applications. The study was published on July 27 in Nature Materials.