Particles could be in a superposition of different branes, say physicists.


I reported on this finding which the National Labs in Oak Ridge TN published yesterday. This is MIT’s own report on the research and discovery of new material called bismuth selenide (Bi2Se3) with an ultrathin layer of a magnetic material, europium sulfide (EuS). I know that is a mouth full. However, the end result will be that it could lead to a new generation of electronics, spintronics, or quantum computing devices. Definitely a big move forward in bridging QC into all things that use daily.
A new and unexpected magnetic effect has taken researchers by surprise, and could open up a new pathway to advanced electronic devices and even robust quantum computer architecture.
The finding is based on a family of materials called topological insulators (TIs) that has drawn much interest in recent years. The novel electronic properties of TIs might ultimately lead to new generations of electronic, spintronic, or quantum computing devices. The materials behave like ordinary insulators throughout their interiors, blocking electrons from flowing, but their outermost surfaces are nearly perfect conductors, allowing electrons to move freely. The confinement of electrons to this vanishingly thin surface makes then behave in unique ways.
But harnessing the materials’ promise still faces numerous obstacles, one of which is to find a way of combining a TI with a material that has controllable magnetic properties. Now, researchers at MIT and elsewhere say they have found a way to overcome that hurdle.
Subscribe! Receive a convenient email notification whenever a new Nanowerk Nanotechnology Spotlight posts.
Become a Spotlight guest author! Have you just published a scientific paper or have other exciting developments to share with the nanotechnology community? Here is how to publish on nanowerk.com.
Creating Q-Dots/ QDs (Acronym seems to depend on which reference book, article that you read) more cheaply and efficiently too.
Quantum dots (QDs) are semiconducting nanocrystals prized for their optical and electronic properties. The brilliant, pure colors produced by QDs when stimulated with ultraviolet light are ideal for use in flat screen displays, medical imaging devices, solar panels and LEDs. One obstacle to mass production and widespread use of these wonder particles is the difficulty and expense associated with current chemical manufacturing methods that often requiring heat, high pressure and toxic solvents.
But now three Lehigh University engineers have successfully demonstrated the first precisely controlled, biological way to manufacture quantum dots using a single-enzyme, paving the way for a significantly quicker, cheaper and greener production method. Their work was recently featured in an article in The New York Times called “A curious tale of quantum dots.”
The Lehigh team— Bryan Berger, Class of 1961 Associate Professor, Chemical and Biomolecular Engineering; Chris Kiely, Harold B. Chambers Senior Professor, Materials Science and Engineering and Steven McIntosh, Class of 1961 Associate Professor, Chemical and Biomolecular Engineering, along with Ph.D. candidate Li Lu and undergraduate Robert Dunleavy—have detailed their findings in an article called “Single Enzyme Biomineralization of Cadmium Sulfide Nanocrystals with Controlled Optical Properties” published in the Proceedings of the National Academy of Sciences (PNAS).
I personally can confirm that QC is not being worked on and advance by just a couple groups such as D-Wave and IBM. The questions/bumps in the road that we will all face is threefold:
1) how do we standardize the QC? right now (like most innovation) is done in siloes and limited cross-collaboration across government, labs & universities, and commercial companies. 2) governance and compliance; how will these need to change across multiple areas 3) id & mitigate all impacts instead of after deployment (don’t be reactive) because we will not have that luxury due to hackers.
There is a temptation to lump quantum computing in with technologies such as fusion power in the sense that both have been proposed for decades with the promise of tremendous leaps in performance.
Whilst fusion power continues to frustrate, there are signs of real progress being made in quantum computing. There is barely a tech giant in the world that doesn’t have dedicated teams working on the topic, and these teams are beginning to bring quantum computing out of the lab and into the real world.
At the forefront of this is IBM, who recently announced that they would connect up a quantum computer to the web and allow us to play with it. The project involves a 5 qubit machine, with a qubit allowing it to operate in both ‘0 and 1’ states at the same time, thus increasing its potential computational power enormously. A one qubit machine has roughly 16 possible states, but once you get over 300, you begin to exceed the number of atoms in the universe.
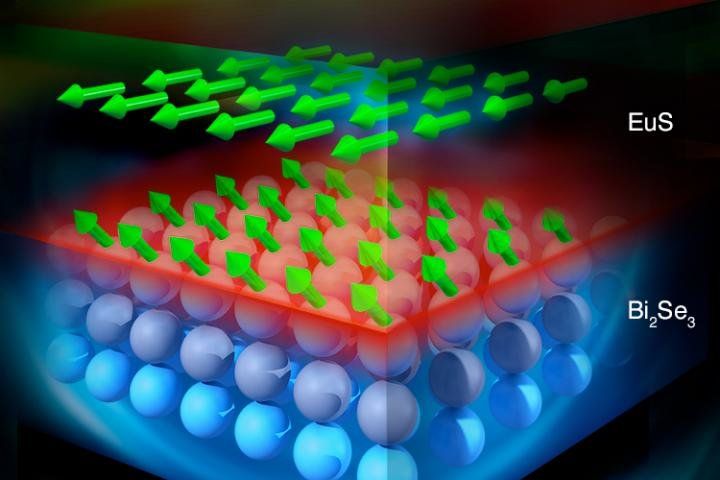
I know that I reported on this a few weeks ago; however, this article shares some additional insights on how this new method will enable more efficient smaller devices including promoting stabilization in Quantum Computing (QC)…
A multi-institutional team of researchers has discovered novel magnetic behavior on the surface of a specialized material that holds promise for smaller, more efficient devices and other advanced technology.
Researchers at the Department of Energy’s Oak Ridge National Laboratory, Massachusetts Institute of Technology and their collaborators used neutron scattering to reveal magnetic moments in hybrid topological insulator (TI) materials at room temperature, hundreds of degrees Fahrenheit warmer than the extreme sub-zero cold where the properties are expected to occur.
The discovery promises new opportunities for next-generation electronic and spintronic devices such as improved transistors and quantum computing technologies. Their research is discussed in a paper published in the journal Nature.
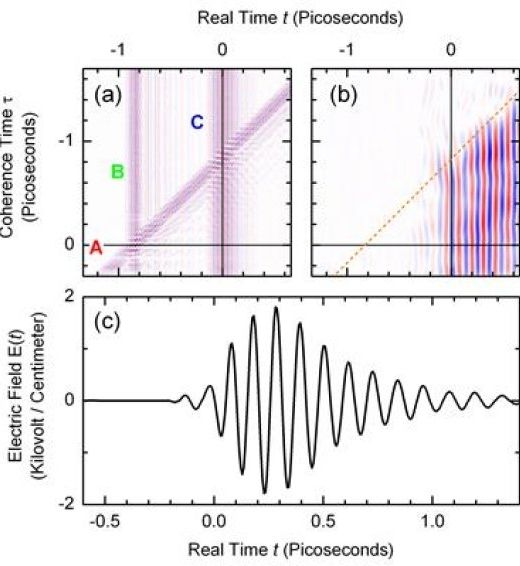
One of those freaky states of Quantum. Wild.
Two-quantum oscillations of atoms in a semiconductor crystal are excited by ultrashort terahertz pulses. The terahertz waves radiated from the moving atoms are analyzed by a novel time-resolving method and demonstrate the non-classical character of large-amplitude atomic motions.
The classical pendulum of a clock swings forth and back with a well-defined elongation and velocity at any instant in time. During this motion, the total energy is constant and depends on the initial elongation which can be chosen arbitrarily. Oscillators in the quantum world of atoms and molecules behave quite differently: their energy has discrete values corresponding to different quantum states. The location of the atom in a single quantum state of the oscillator is described by a time-independent wavefunction, meaning that there are no oscillations.
Oscillations in the quantum world require a superposition of different quantum states, a so-called coherence or wavepacket. The superposition of two quantum states, a one-phonon coherence, results in an atomic motion close to the classical pendulum. Much more interesting are two-phonon coherences, a genuinely non-classical excitation for which the atom is at two different positions simultaneously. Its velocity is nonclassical, meaning that the atom moves at the same time both to the right and to the left as shown in the movie. Such motions exist for very short times only as the well-defined superposition of quantum states decays by so-called decoherence within a few picoseconds (1 picosecond = 10-12 s). Two-phonon coherences are highly relevant in the new research area of quantum phononics where tailored atomic motions such as squeezed and/or entangled phonons are investigated.
(Phys.org)—Animal muscle needs to be strong enough to endure strain; it must also be flexible and elastic; and it is self-healing. Finding a polymer that has all of these properties has proved challenging. However, researchers from Stanford, Nanjing University, UC Riverside, Harvard, and the University of Colorado have reported the synthesis of an elastomer that mimics the properties of animal muscle. Their polymer, is also stable at room temperature and not sensitive to water. Their work appears in Nature Chemistry.
Efforts to create polymers that mimic the properties of biological muscle have come short of being practically useful. Often the bonding involved in making these polymers must be sufficiently strong to serve as actuators, but weak enough for reversible self-healing. Many models, to date, involve hydrogen bonding, but hydrogen bonds are sensitive to water. Li, et al. have, instead, exploited metal-ligand interactions as a way to mimic muscle properties.
The ligand 2,6-pyridinedicarboxamide (pdca)binds to Fe(III) via the pyridyl nitrogen and the nitrogen and oxygen on the carboxamides. Two pdca molecules coordinate to one Fe(III) atom through six coordination sites. Two of the sites are strong bonds (the pyridyl), two sites are “medium” strength bonds (the amides), and two are weak bonds (the carboxyl). Calculations of bond strength show that the strong bonds are similar to covalent bonds, while the weak Fe-O bonds are similar to hydrogen bonding. This multi-bonding structure, as it turns out, provides an excellent framework for making an elastomer.
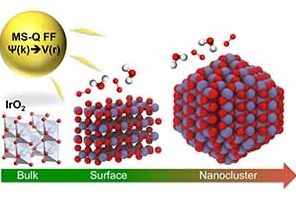
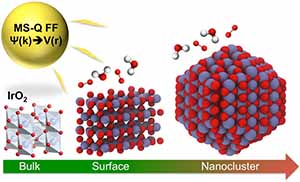
Iridium oxide (IrO2) nanoparticles are useful electrocatalysts for splitting water into oxygen and hydrogen — a clean source of hydrogen for fuel and power. However, its high cost demands that researchers find the most efficient structure for IrO2 nanoparticles for hydrogen production.
A study conducted by a team of researchers at the U.S. Department of Energy’s (DOE’s) Argonne National Laboratory, published in Journal of Materials Chemistry A, describes a new empirical interatomic potential that models the IrO2 properties important to catalytic activity at scales relevant to technology development. Also known as a force field, the interatomic potential is a set of values describing the relationship between structure and energy in a system based on its configuration in space. The team developed their new force field based on the MS-Q force field.
“Before, it was not possible to optimize the shape and size of a particle, but this tool enables us to do this,” says Maria Chan, assistant scientist at Argonne’s Center for Nanoscale Materials (CNM), a DOE Office of Science User Facility.