The XENON experiment recently made a breakthrough in their hunt for dark matter, observing the most rare decay process in the Universe that involves neutrinos.


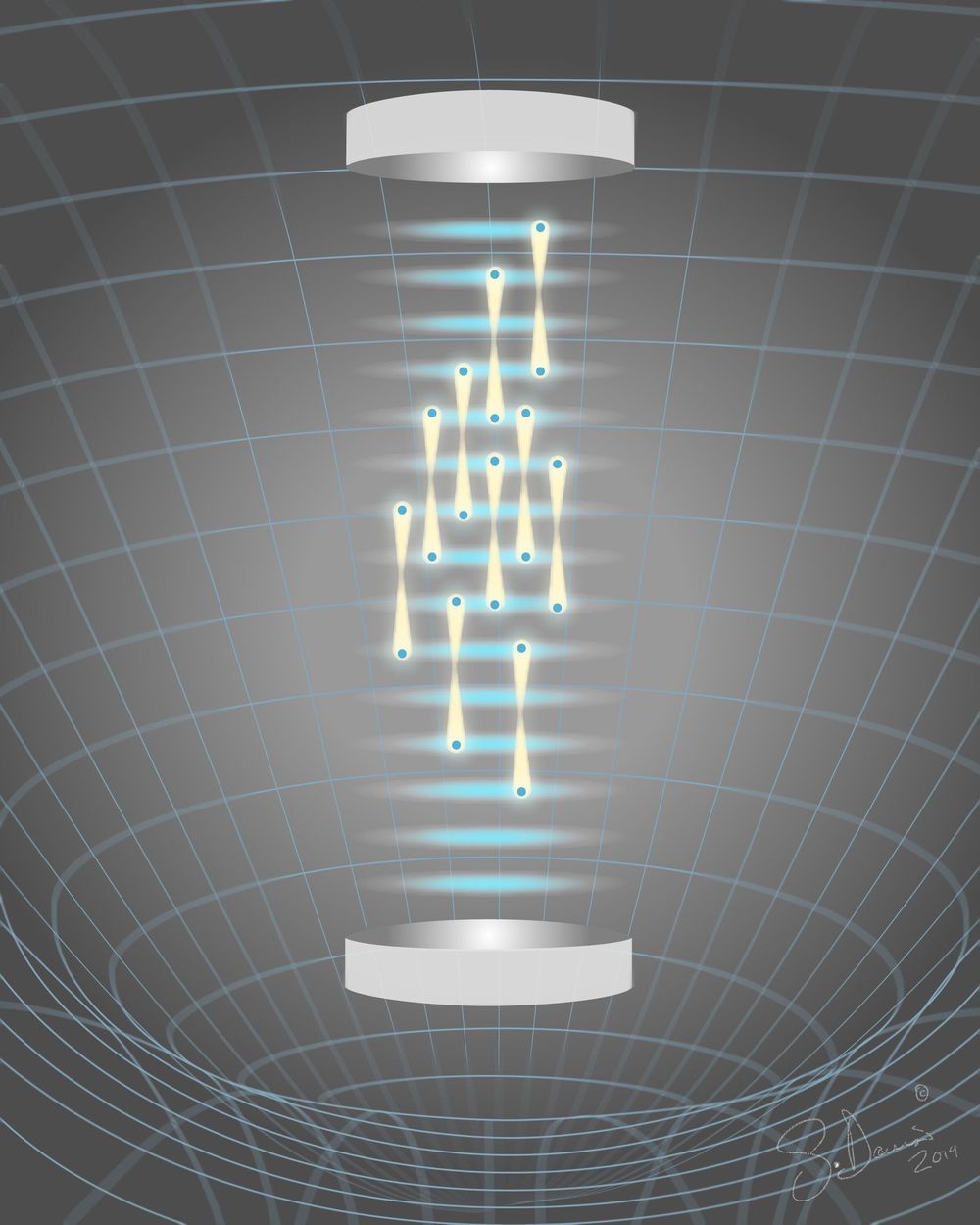
A team of researchers at the University of California, Berkeley, has found a new way to measure gravity—by noting differences in atoms in a supposition state, suspended in the air by lasers. In their paper published in the journal Science, the group describes their new technique and explain why they believe it will be more useful than traditional methods.
Currently, the standard way to conduct gravity experiments is to drop things down shielded tubes and measure them as they whiz by instruments. In addition to giving researchers a very brief glimpse of gravitational interactions, such methods often fall prey to inadvertent stray magnetic fields. In this new effort, the researchers have found a way to measure gravity that does not involve dropping objects at all.
The new approach involved releasing a cloud of cesium atoms into the air in a small chamber and then using flashing lights to split several of them into a superposition state. Once split, lasers were used to keep all the atoms in fixed positions with one of each pair raised slightly higher than its mate. The team then measured each atom’s wave particle duality, which is impacted by gravity. By measuring the difference in duality between the paired atoms (because of the difference in their distances from Earth), the researchers were able to come up with a measurement for gravity.
A newly developed laser technology has enabled physicists in the Laboratory for Attosecond Physics (jointly run by LMU Munich and the Max Planck Institute of Quantum Optics) to generate attosecond bursts of high-energy photons of unprecedented intensity. This has made it possible to observe the interaction of multiple photons in a single such pulse with electrons in the inner orbital shell of an atom.
In order to observe the ultrafast electron motion in the inner shells of atoms with short light pulses, the pulses must not only be ultrashort, but very bright, and the photons delivered must have sufficiently high energy. This combination of properties has been sought in laboratories around the world for the past 15 years. Physicists at the Laboratory for Attosecond Physics (LAP), a joint venture between the Ludwig-Maximilians-Universität Munich (LMU) and the Max Planck Institute of Quantum Optics (MPQ), have now succeeded in meeting the conditions necessary to achieve this goal. In their latest experiments, they have been able to observe the non-linear interaction of an attosecond pulse with electrons in one of the inner orbital shells around the atomic nucleus. In this context, the term ‘non-linear’ indicates that the interaction involves more than one photon (in this particular case two are involved).


Circa 2016
Laser physicists in Munich have measured a photoionization — in which an electron exits a helium atom after excitation by light — for the first time with zeptosecond precision. A zeptosecond is a trillionth of a billionth of a second (10^−21 seconds). This is the greatest accuracy of time determination ever achieved, as well as the first absolute determination of the timescale of photoionization.
If light hits the two electrons of a helium atom, one must be incredibly fast to observe what occurs. Besides the ultra-short periods in which changes take place, quantum mechanics also comes into play. Laser physicists at the Max Planck Institute of Quantum Optics (MPQ), the Technical University of Munich (TUM) and the Ludwig Maximilians University (LMU) Munich have now measured such an event for the first time with zeptosecond precision.
Either the entire energy of a light particle (photon) can be absorbed by one of the electrons or a division can take place, if a photon hits the two electrons of a helium atom. Regardless of the energy transfer, one electron leaves the atom. This process is called photoemission, or photoelectric effect, and was explained by Albert Einstein at the beginning of last century.
In 1884, a schoolmaster and theologian named Edwin Abbott wrote a novella called Flatland, which tells the story of a world populated by sentient two-dimensional shapes. While intended as a satire of rigid Victorian social norms, Flatland has long fascinated mathematicians and physicists and served as the setting for many a thought experiment.
One such thought experiment: How can light be controlled in two dimensions?
When a wave of light is confined on a two-dimensional plane by certain materials, it becomes something known as a polariton—a particle that blurs the distinction between light and matter. Polaritons have exciting implications for the future of optical circuits because, unlike electronic integrated circuits, integrated optics is difficult to miniaturize with commonly used materials. Polaritons allow light to be tightly confined to the nanoscale, even potentially to the thickness of a few atoms.
The second objective is propulsion. This is achieved by emitting pulsed cathode rays out of one end of the craft tuned to the rate of change of jet stream particles surrounding the bubble. At the other end of the craft, cations are emitted at the same rate of change. This creates a push/pull effect, doubling the ship’s acceleration and velocity capabilities.
O.o.
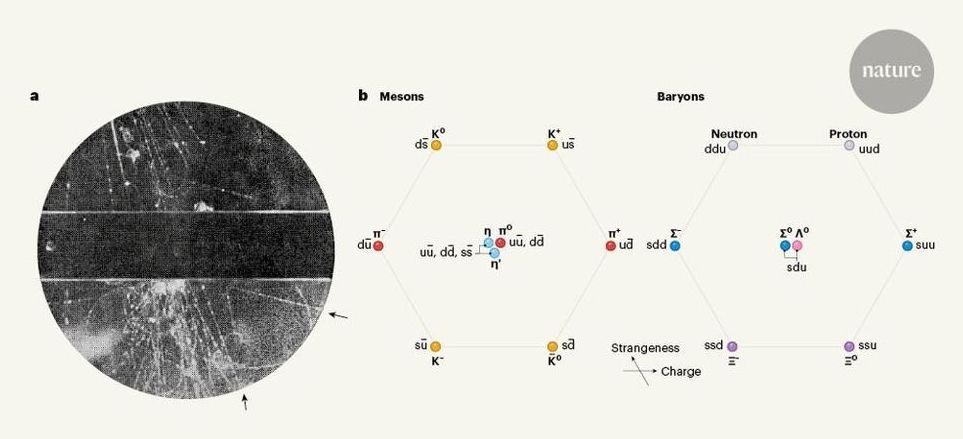
In 1947, scientists found a previously unseen particle, which is now called a neutral kaon. This work led to the discovery of elementary particles known as quarks, and ultimately to the establishment of the standard model of particle physics. From the observation of a neutral kaon to the standard model.
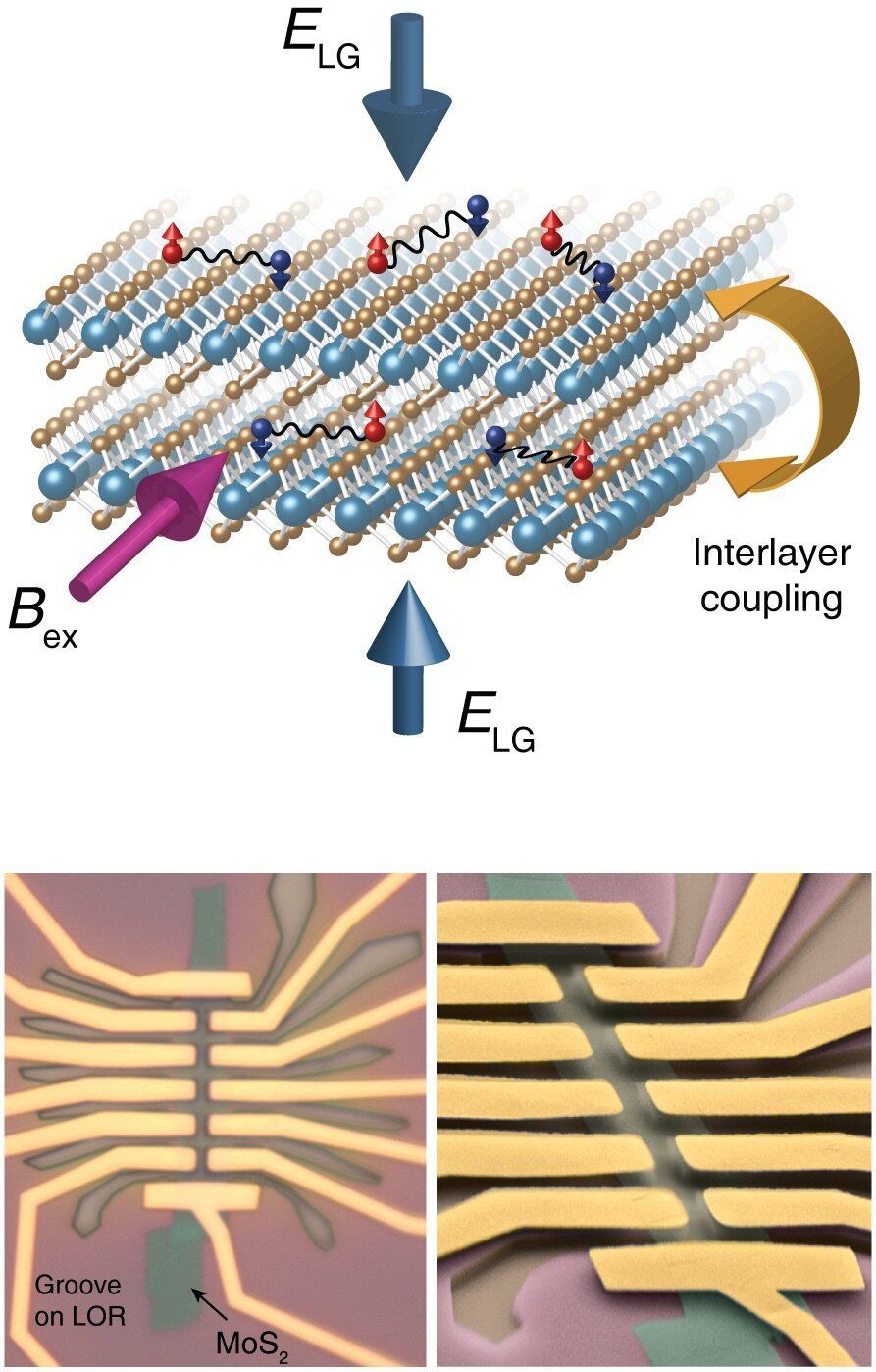
In superconducting materials, an electric current will flow without any resistance. There are quite a few practical applications of this phenomenon; however, many fundamental questions remain as yet unanswered. Associate Professor Justin Ye, head of the Device Physics of Complex Materials group at the University of Groningen, studied superconductivity in a double layer of molybdenum disulfide and discovered new superconducting states. The results were published in the journal Nature Nanotechnology on 4 November.
Superconductivity has been shown in monolayer crystals of, for example, molybdenum disulphide or tungsten disulfide that have a thickness of just three atoms. “In both monolayers, there is a special type of superconductivity in which an internal magnetic field protects the superconducting state from external magnetic fields,” Ye explains. Normal superconductivity disappears when a large external magnetic field is applied, but this Ising superconductivity is strongly protected. Even in the strongest static magnetic field in Europe, which has a strength of 37 Tesla, the superconductivity in tungsten disulfide does not show any change. However, although it is great to have such strong protection, the next challenge is to find a way to control this protective effect, by applying an electric field.
Too large to be classed as molecules, but too small to be bulk solids, atomic clusters can range in size from a few dozen to several hundred atoms. The structures can be used for a diverse range of applications, which requires a detailed knowledge of their shapes. These are easy to describe using mathematics in some cases; while in others, their morphologies are far more irregular. However, current models typically ignore this level of detail; often defining clusters as simple ball-shaped structures.
In research published in The European Physical Journal B, José M. Cabrera-Trujillo and colleagues at the Autonomous University of San Luis Potosí in Mexico propose a new method of identifying the morphologies of atomic clusters. They have now confirmed that the distinctive geometric shapes of some clusters, as well as the irregularity of amorphous structures, can be fully identified mathematically.
The insights gathered by Cabrera-Trujillo’s team could make it easier for researchers to engineer atomic clusters for specific applications. These could include nanoparticles containing two different metals, which are highly effective in catalysing chemical reactions. Their updated methods provided new ways to determine the structural properties of clusters, the ways in which they convert energy to different forms, and the potential forces between atoms. The technique was also able to distinguish the surrounding environments of atoms in the cores of clusters, and on their surfaces. Ultimately, this allowed the researchers to distinguish between distinctive shapes, including icosahedrons, octahedrons, and simple pancakes. They were also able to identify amorphous shapes, which contain no discernible mathematical order.