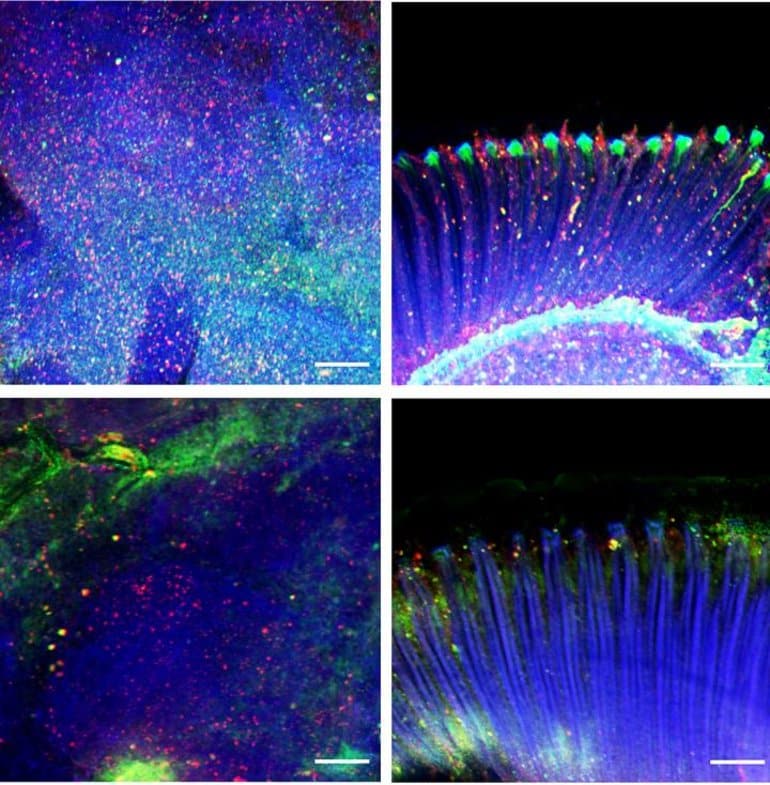
More than 25 years ago, biologists in Arkansas began to report dozens of bald eagles paralyzed, convulsing, or dead. Their brains were pocked with lesions never seen before in eagles. The disease was soon found in other birds across the southeastern United States. Eventually, researchers linked the deaths to a new species of cyanobacteria growing on an invasive aquatic weed that is spreading across the country. The problem persists, with the disease detected regularly in a few birds, yet the culprit’s chemical weapon has remained unknown.
Today in Science, a team identifies a novel neurotoxin produced by the cyanobacteria and shows that it harms not just birds, but fish and invertebrates, too. “This research is a very, very impressive piece of scientific detective work,” says microbiologist Susanna Wood of the Cawthron Institute. An unusual feature of the toxic molecule is the presence of bromine, which is scarce in lakes and rarely found in cyanobacteria. One possible explanation: the cyanobacteria produce the toxin from a bromide-containing herbicide that lake managers use to control the weed.
The discovery highlights the threat of toxic cyanobacteria that grow in sediment and on plants, Wood says, where routine water quality monitoring might miss them. The finding also equips researchers to survey lakes, wildlife, and other cyanobacteria for the new toxin. “It will be very useful,” says Judy Westrick, a chemist who studies cyanobacterial toxins at Wayne State University and was not involved in the new research. “I started jumping because I got so excited.”