Remembering life experiences from the visual perspective of an outside observer changes the brain’s functional connectivity and appears to make traumatic memories less intense.


The 19 hospitalized patients from a single nursing home had tested positive for the virus in April.
All were older than age 64 and had chronic health conditions such as hypertension, dementia, heart disease, diabetes and lung diseases. Their age and health issues are considered risk factors for severe COVID-19.
The patients received either one or two intravenous doses of itolizumab along with the standard treatments used in Cuba at the time. Those included antiviral drugs, antibiotics, chloroquine, interferon, and blood thinners. Only two patients required oxygen therapy after the first dose, and all but one were discharged from the hospital in 14 days.
N”(Reuters) — An antibody drug already being used against the coronavirus in Cuba decreased the risk of intensive care admission and death among nursing home residents with moderate COVID-19, according to a small study conducted in the island nation.
India’s Biocon Ltd said earlier this month it received regulatory approval in India for itolizumab for use in coronavirus infected patients with moderate-to-severe respiratory distress. It was originally tested as a treatment for psoriasis.
Researchers, including from Cuba’s Center of Molecular Immunology, which developed itolizumab, said timely use of the drug in combination with standard therapy helped reduce inflammation and prevented COVID-19 from worsening.
Scientists from 4 different Swiss universities describe how adhesion molecules activate autoaggressive immune cells and drive their infiltration in the nervous system in a model of multiple sclerosis.
Click to read the paper published in Frontiers in Immunology: https://fro.ntiers.in/tp1U
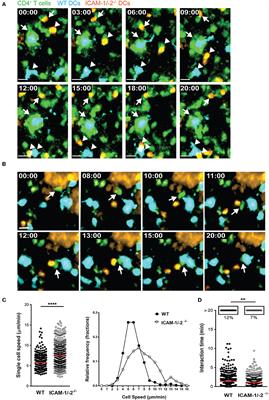
In experimental autoimmune encephalomyelitis (EAE), an animal model of multiple sclerosis (MS), myelin-specific T cells are activated in the periphery and differentiate in T helper (Th) 1 and Th17 effector cells, which cross the blood-brain barrier (BBB) to reach the central nervous system (CNS), where they induce neuroinflammation. Here, we explored the role of intercellular adhesion molecule-1 (ICAM-1) and ICAM-2 in the activation of naïve myelin-specific T cells and in the subsequent migration of differentiated encephalitogenic Th1 and Th17 cells across the BBB in vitro and in vivo. While on antigen-presenting cells ICAM-1, but not ICAM-2 was required for the activation of naïve CD4+ T cells, endothelial ICAM-1 and ICAM-2 mediated both Th1 and Th17 cell migration across the BBB. ICAM-1/-2-deficient mice developed ameliorated typical and atypical EAE transferred by encephalitogenic Th1 and Th17 cells, respectively. Our study underscores important yet cell-specific contributions for ICAM-1 and ICAM-2 in EAE pathogenesis.
Multiple sclerosis (MS) is considered an autoimmune inflammatory demyelinating disease of the central nervous system (CNS). Experimental autoimmune encephalomyelitis (EAE), a prototypic animal model for MS, mimics many aspects of the acute inflammatory phase of the human disease (1). In EAE, naïve myelin-reactive CD4+ T cells are activated and differentiated in peripheral lymphoid tissue into encephalitogenic Th1 or Th17 cells, which travel in the blood circulation to the CNS. After crossing the blood-brain barrier (BBB) they next infiltrate in the CNS parenchyma, leading to clinical manifestation of the disease (2). EAE can be actively induced by immunization with CNS myelin antigens emulsified in complete Freund’s adjuvant (aEAE) or by injection of myelin-reactive CD4+ T cells into syngeneic naïve recipients (tEAE) (3, 4).
Activation of naïve CD4+ T cells during aEAE occurs in the draining peripheral lymph nodes (dLNs), where T cells recognize their cognate antigen (Ag) on antigen-presenting cells (APCs) forming periodic contacts between the T-cell receptor (TCR) and the myelin oligodendrocyte glycoprotein (MOG)aa35−55 peptide loaded major histocompatibility complex (pMHC) on the APCs, referred to as the immunological synapse (IS) (5, 6). The fate of naïve T cells is determined within hours after Ag exposure by interacting with APCs in LN. The interaction between the integrin lymphocyte function associated antigen-1 (LFA-1) on the T cells and its ligand intercellular adhesion molecule-1 (ICAM-1) on the APCs is suggested to be involved in modulating the IS. However, APCs additionally express ICAM-2, an alternate ligand of LFA-1.

COSTA MESA, Calif. – Health experts say around half of American adults are overweight or obese. While excessive body weight is linked to a number of serious health conditions, including diabetes and heart disease, a new study reveals it can also reduce blood flow to the brain. Researchers warn this can put overweight individuals at great risk for Alzheimer’s disease.
The study examines brain blood flow in 17,721 adults between 18 and 94. To do this, researchers use a brain imaging technique known as single photon emission computed tomography (SPECT).
SPECT is a technique in which doctors inject a radioactive tracer into a patient’s blood and then use a special camera to look at the flow of blood. Participants were then split into five categories: underweight, normal weight, overweight, obese, and morbidly obese — to examine blood flow in each of their brains. The brain scan data reveals lower blood flow across virtually all brain regions as body weight increases.

Some neuroscientists are daring to wield the technologies to probe one powerful group of internal brain states: emotions. Others are applying them to states such as motivation, or existential drives such as thirst. Researchers are even finding signatures of states in their data for which they have no vocabulary.
Neuroscientists are scrutinizing huge piles of data to learn how brains create emotions and other internal states such as aggression and desire.


The answer is in their genes—especially those that encode for basic life functions, such as metabolism. Thanks to the lowly C. elegans worm, we’ve uncovered genes and molecular pathways, such as insulin-like growth factor 1 (IGF-1) signaling that extends healthy longevity in yeast, flies, and mice (and maybe us). Too nerdy? Those pathways also inspired massive scientific and popular interest in metformin, hormones, intermittent fasting, and even the ketogenic diet. To restate: worms have inspired the search for our own fountain of youth.
Still, that’s just one success story. How relevant, exactly, are those genes for humans? We’re rather a freak of nature. Our aging process extends for years, during which we experience a slew of age-related disorders. Diabetes. Heart disease. Dementia. Surprisingly, many of these don’t ever occur in worms and other animals. Something is obviously amiss.
In this month’s Nature Metabolism, a global team of scientists argued that it’s high time we turn from worm to human. The key to human longevity, they say, lies in the genes of centenarians. These individuals not only live over 100 years, they also rarely suffer from common age-related diseases. That is, they’re healthy up to their last minute. If evolution was a scientist, then centenarians, and the rest of us, are two experimental groups in action.
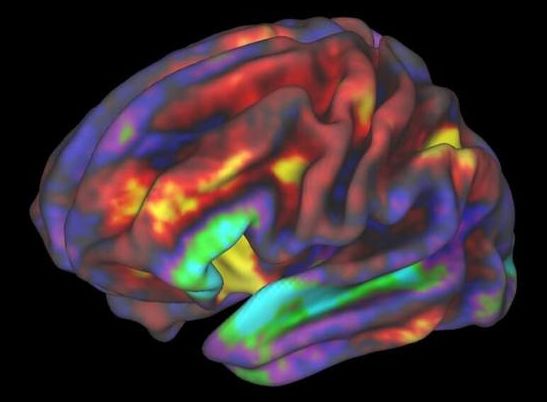
But have you ever wondered: how well do those maps represent my brain? After all, no two brains are alike. And if we’re ever going to reverse-engineer the brain as a computer simulation—as Europe’s Human Brain Project is trying to do—shouldn’t we ask whose brain they’re hoping to simulate?
Enter a new kind of map: the Julich-Brain, a probabilistic map of human brains that accounts for individual differences using a computational framework. Rather than generating a static PDF of a brain map, the Julich-Brain atlas is also dynamic, in that it continuously changes to incorporate more recent brain mapping results. So far, the map has data from over 24,000 thinly sliced sections from 23 postmortem brains covering most years of adulthood at the cellular level. But the atlas can also continuously adapt to progress in mapping technologies to aid brain modeling and simulation, and link to other atlases and alternatives.
In other words, rather than “just another” human brain map, the Julich-Brain atlas is its own neuromapping API—one that could unite previous brain-mapping efforts with more modern methods.

Last year information was released concerning rejuvenation of the thymus which resulted in a reversal of the epigenetic clock an average of 2.5 years in a small trial of 9 people costing $10,000 per person. You can get this done too. A company has formed called Intervene Immune which will take on volunteers for the process. It is not funded so you would have to pay out pf pocket though eventually the cost may come down and they can provide financing. You do not have to travel to California to get this done. Cost prohibits me, and I may or may not be eligible as I have IBS though that is not on the exclusion list. I emailed them concerning all this which is how I got the information.
http://interveneimmune.com/
https://www.surveymonkey.com/r/TRIIMX
The TRIIM-X trial is an expanded pilot clinical study that will evaluate a personalized combination treatment regimen for thymus regeneration. The thymus is a part of the immune system that declines markedly with age, and regenerating it may prevent or reverse key aspects of immunosenescence (immune system aging) and potentially prevent or reverse key parts of the aging process more generally. The study will evaluate biomarkers for epigenetic aging and immunosenescence, as well as evaluate established clinical measures and risk factors for prevention of physical frailty, cancer, cardiovascular disease, diabetes, dementia, and also infectious diseases, including flu and COVID-19.
The study uses multiple agents in combination with personalized doses of recombinant human growth hormone (somatropin), metformin, and DHEA, in a similar manner to how the combination treatment was applied in the earlier TRIIM trial at Stanford, which demonstrated strong statistical significance for the primary efficacy endpoints that will be evaluated in TRIIM-X. Somatropin is approved by the FDA for adult growth hormone deficiency and its use in the study is guided by prior safety data established for that use and also based on safety data available on its prior use in the TRIIM trial and in clinical practice in healthy elderly individuals. There will also be control groups that enable testing of biomarker variability and the contribution of individual medications within the combination treatment.
The objective of the study is to obtain information needed for designing an effective personalized and adaptive treatment regimen for a larger and more diverse study population, and to obtain additional proof of principle for the new use of the medications and biomarkers for preventive medicine. The duration of treatment in the TRIIM-X trial will be 12 months.