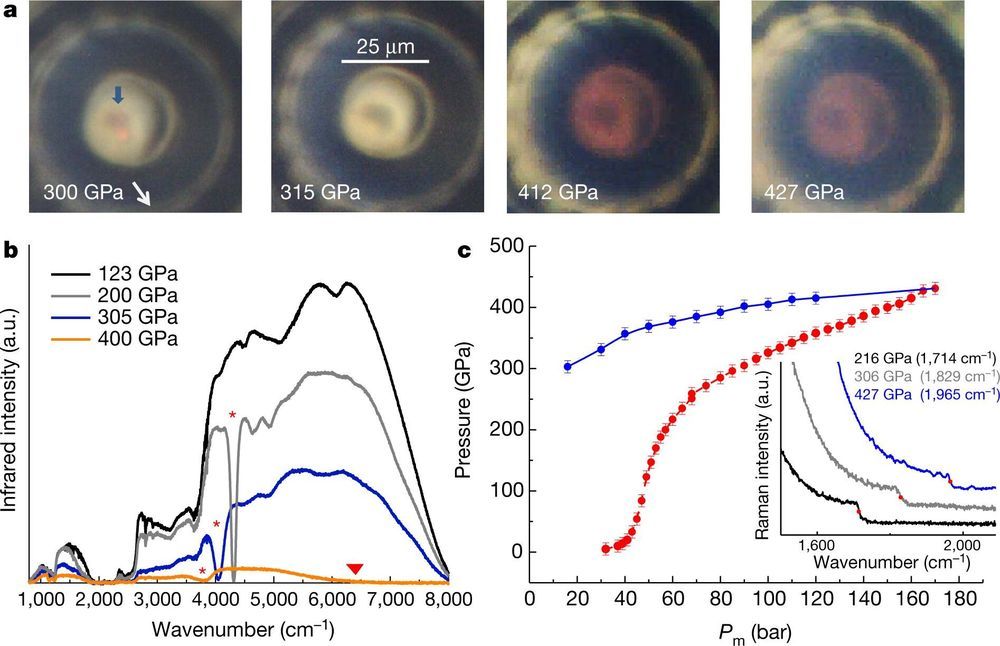
A team of researchers, two with the French Atomic Energy Commission (AEC) and a third with the Soleil synchrotron, have found evidence of a phase change for hydrogen at a pressure of 425 gigapascals. In their paper published in the journal Nature, Paul Loubeyre, Florent Occelli and Paul Dumas describe testing hydrogen at such a high pressure and what they learned from it.
Researchers long ago theorized that if hydrogen gas were exposed to enough pressure, it would transition into a metal. But the theories were not able to derive how much pressure is required. Doubts about the theories began to arise when scientists developed tools capable of exerting the high pressures that were believed necessary to squeeze hydrogen into a metal. Theorists simply moved the number higher.
In the past several years, however, theorists have come to a consensus—their math showed that hydrogen should transition at approximately 425 gigapascals—but a way to generate that much pressure did not exist. Then, last year, a team at the AEC improved on the diamond anvil cell, which for years has been used to create intense pressure in experiments. In a diamond anvil cell, two opposing diamonds are used to compress a sample between highly polished tips—the pressure generated is typically measured using a reference material. With the new design, called a toroidal diamond anvil cell, the tip was made into a donut shape with a grooved dome. When in use, the dome deforms but does not break at high pressures. With the new design, the researchers were able to exert pressures up to 600 GPa. That still left the problem of how to test a sample of hydrogen as it was being squeezed.