In regenerative medicine, an ideal treatment for patients whose muscles are damaged from lack of oxygen would be to invigorate them with an injection of their own stem cells.


I hope you can take the time to watch my interview with Nicolas Chernavsky & Nina Torres Zanvettor where we discuss their rejuvenation translation company NTZ Publicações and why we need more rejuvenation translators to join their team to spread rejuvenation science globally to a mainstream audience.
https://instagram.com/brent.nally/ https://facebook.com/brent.nally https://linkedin.com/in/brentnally https://twitter.com/BrentNally https://patreon.com/BrentNally https://dlive.tv/BrentNally https://gab.com/BrentNally https://medium.com/@brentnally https://twitch.tv/brentnally https://brentnally.tumblr.com/ https://reddit.com/user/BrentNally Discord Brent Nally#0616 TikTok @brentnally Telegram @BrentNally Snapchat brentnally Mixer BrentNally https://levscience.com/
- My mission is to drastically improve your life by helping you break bad habits, build and keep new healthy habits to make you the best version of yourself.
- Please consider donating: https://paypal.me/BrentNally or my Bitcoin Cash (BCH) address: qr9gcfv92pzwfwa5hj9sqk3ptcnr5jss2g78n7w6f2 or Patreon
- Please also subscribe and hit the notification bell and click “all” on these YouTube channels:
https://youtube.com/user/LifespanIO/videos https://youtube.com/channel/UCL6WcAha_oYrl5johM6PwuQ
https://youtube.com/channel/UCAvRKtQNLKkAX0pOKUTOuzw
https://youtube.com/user/EternalLifeFan
Huge thanks to Roen Horn for editing this video!
Intro & exit song by soundsage.
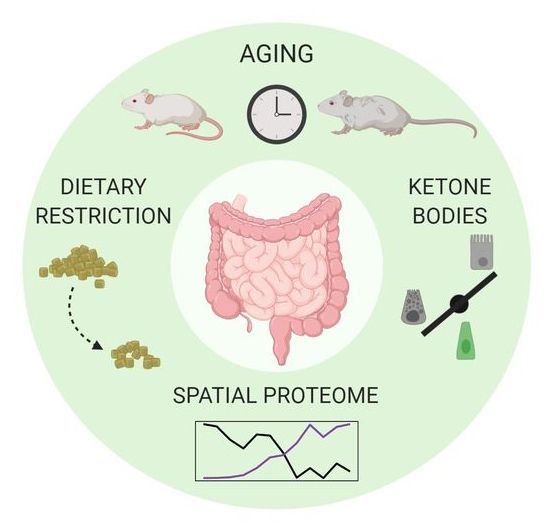
The small intestine is one of the most important interfaces between the environment and our body. It is responsible for nutrient absorption but also forms a barrier against potentially harmful environmental cues. A research team, led by researchers from the Leibniz Institute on Aging—Fritz Lipmann Institute (FLI) in Jena, Germany, investigated the effects of aging and diet on the intestinal epithelium of young and old mice. They were able to show region-specific effects on the proteome and age-related impairments in adaptation to nutrient availability. Their results provide a complete picture of the spatial organization of the small intestine proteome in the mouse. The results were published in the journal Cell Reports.
The small intestine (SI) is one of the most important interfaces between the environment and our body. It has two major functions: it is responsible for absorbing nutrients from the food we eat and functions as a barrier to restrict the entry of harmful substances. The SI is a highly adaptive and dynamic organ, as it adapts to changes in nutrient intake or diet. The intestinal epithelium undergoes a process of continuous renewal, every 3–5 days.
Effects of aging and diet on the small intestine have already been investigated. It is known that aging leads to reduced absorption of nutrients by the epithelium thus contributing to malnutrition in elderly people. In addition, anatomical differences between different regions of the SI are well known, but so far, region-specific effects of aging and diet on the set of proteins that compose the intestinal epithelium had not been investigated.
Liz Parrish, founder and CEO of BioViva USA Inc., together with Dr. Sewell from Integrated Health Systems (IHS), interviewed by James Strole, Director of the Coalition for Radical Life Extension (CRLE) and Co-Founder of People Unlimited Inc., and Joe Bardin, Communications Director of the CRLE.
The interview took place on April 25, 2020.
The Coalition of Radical Life Extension are de producers of the anual event RAADfest (Revolution Against Aging and Death Festival) which will take place this year in Las Vegas, October 1 — 4. I highly recommend you consider attending this event so take a look at https://www.raadfest.com/
To watch the entire webinar for free visit the following link: https://www.rlecoalition.com/webinar
Other related links:
Coalition of Radical Life Extension: https://www.rlecoalition.com/
BioViva: https://biovivascience.myshopify.com/
Integrated Health Systems (IHS): https://www.integrated-health-systems.com/
Join me at any of my media channels:
Youtube: https://www.youtube.com/andresgrases
: https://www.linkedin.com/in/andresgrases/
: https://www.facebook.com/
Instagram: https://www.instagram.com/andgrabri/
Also, visit my own website here: https://transhumanplus.com/

About ColdFusion –
ColdFusion is an Australian based online media company independently run by Dagogo Altraide since 2009. Topics cover anything in science, technology, history and business in a calm and relaxed environment.
If you enjoy my content, please consider subscribing!
I’m also on Patreon: https://www.patreon.com/ColdFusion_TV
Bitcoin address: 13SjyCXPB9o3iN4LitYQ2wYKeqYTShPub8
Script by Fil Zivko
— New Thinking Book written by Dagogo Altraide –
Learn the stories of those who invented the things we use everyday.
Get the book on Amazon: http://bit.ly/NewThinkingbook
Get the book on Google Play: http://bit.ly/NewThinkingGooglePlay
https://newthinkingbook.squarespace.com/about/
— ColdFusion Social Media –
» Twitter | @ColdFusion_TV
» Instagram | coldfusiontv
» Facebook | https://www.facebook.com/ColdFusionTV
— ColdFusion Podcast links –
Google Podcasts — http://bit.ly/2xo8doR
Apple Podcasts — https://apple.co/2WI2IeU
Spotify — https://spoti.fi/2KT1taB
Stitcher — http://bit.ly/2WI4f4E

A group of researchers has demonstrated that treatment with NMN, a precursor of NAD+, restores neurovascular coupling (NVC) in aged mice [1]. Since NVC deficiency seems to be a major factor in the age-related decline of cognitive and motor functions, this discovery presents exciting new possibilities for longevity research.
Neurovascular coupling
While the human brain is the evolutionary advantage that brought us to where we are today, operating this machine requires considerable resources. Our cerebral blood flow (CBF) accounts for 15% of cardiac output and 20% of resting total oxygen consumption, even though the brain itself comprises just 2% of body mass. CBF has to be constantly redirected to the regions of the brain that are currently active, and NVC is the mechanism in charge of this complex operation. Importantly, the CBF/cardiac output ratio decreases with age [2].
Central to a lot of scientific research into aging are tiny caps on the ends of our chromosomes called telomeres. These protective sequences of DNA grow a little shorter each time a cell divides, but by intervening in this process, researchers hope to one day regulate the process of aging and the ill health effects it can bring. A Harvard team is now offering an exciting pathway forward, discovering a set of small molecules capable of restoring telomere length in mice.
Telomeres can be thought of like the plastic tips on the end of our shoelaces, preventing the fraying of the DNA code of the genome and playing an important part in a healthy aging process. But each time a cell divides, they grow a little shorter. This sequence repeats over and over until the cell can no longer divide and dies.
This process is linked to aging and disease, including a rare genetic disease called dyskeratosis congenita (DC). This is caused by the premature aging of cells and is where the team focused its attention, hoping to offer alternatives to the current treatment that involves high-risk bone marrow transplants and which offers limited benefits.
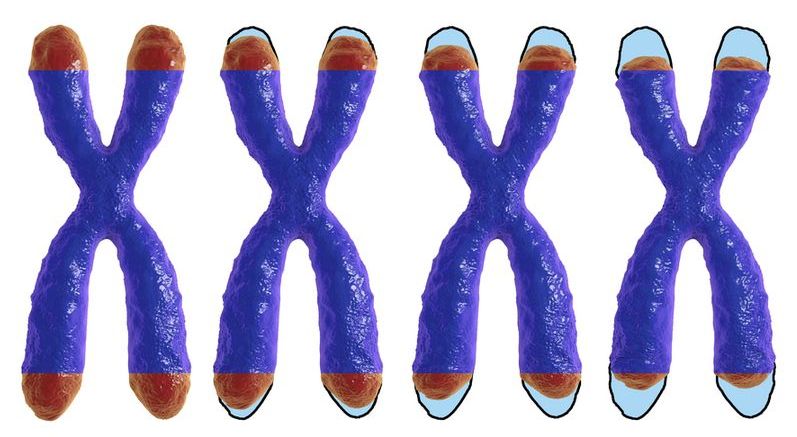
Researchers have demonstrated that it is possible to restore telomerase activity in stem cells affected by telomere biology diseases, which prevent them from producing telomerase and repairing their telomeres.
Telomeres and telomerase
Each chromosome that stores our genetic information has a protective cap at each end known as a telomere, a specific DNA sequence that is repeated thousands of times. This sequence has two purposes: it protects the coding regions of the chromosome and prevents it from being damaged, and it acts as a clock that controls the number of replications a cell can make; this is known as the Hayflick limit.
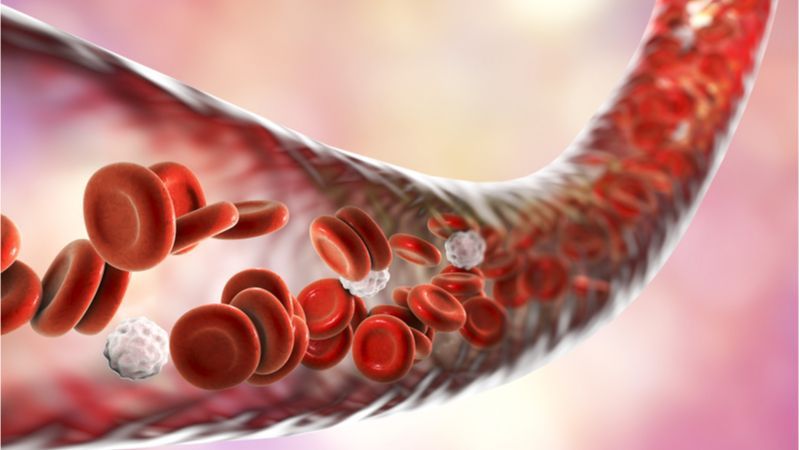
Researchers have developed a way to modify an existing cancer drug with toxic side effects into something that is less toxic to blood platelets and more effective at removing harmful and inflammatory senescent cells, one of the reasons we age, from mice.
What are senescent cells?
As you age, increasing numbers of your cells enter into a state known as senescence. Senescent cells do not divide or support the tissues of which they are part; instead, they emit a range of potentially harmful chemical signals that encourage nearby healthy cells to enter the same senescent state. Their presence causes many problems: they reduce tissue repair, increase chronic inflammation, and can even eventually raise the risk of cancer and other age-related diseases.