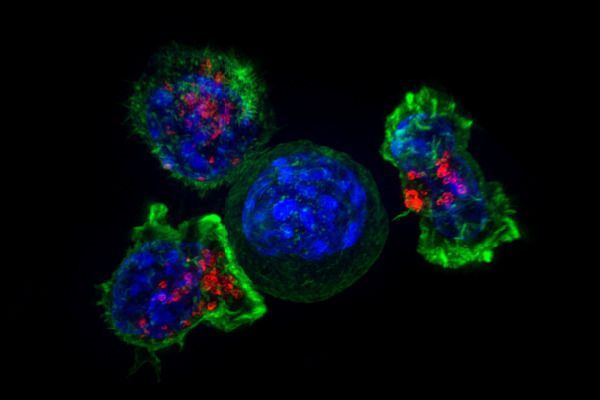
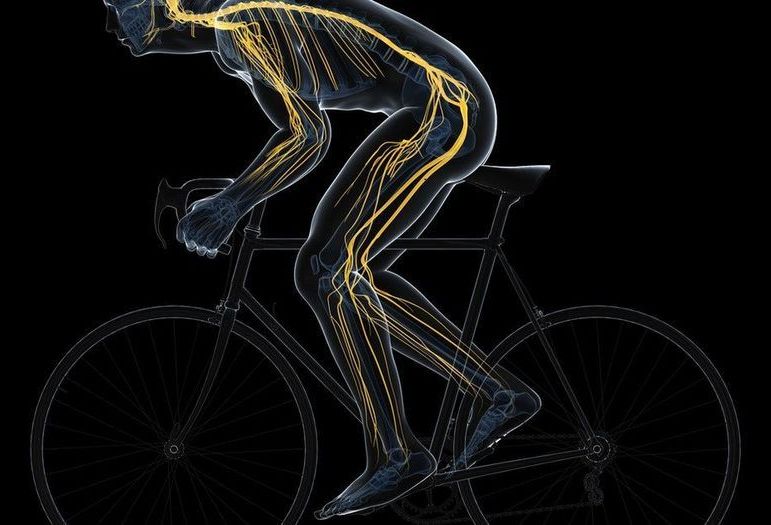
Interventions that may slow ageing include drugs (e.g., rapamycin, metformin), supplements (e.g., nicotinamide riboside, nicotinamide mononucleotide), lifestyle interventions (e.g., exercise) and diets (e.g., fasting)
Thanks to advances in modern medicine over the past century, the world’s population has experienced a marked increase in longevity. However, disparities exist that lead to groups with both shorter lifespan and significantly diminished health, especially in the aged. Unequal access to proper nutrition, healthcare services, and information to make informed health and nutrition decisions all contribute to these concerns. This in turn has hastened the ageing process in some and adversely affected others’ ability to age healthfully. Many in developing as well as developed societies are plagued with the dichotomy of simultaneous calorie excess and nutrient inadequacy. This has resulted in mental and physical deterioration, increased non-communicable disease rates, lost productivity and quality of life, and increased medical costs. While adequate nutrition is fundamental to good health, it remains unclear what impact various dietary interventions may have on improving healthspan and quality of life with age. With a rapidly ageing global population, there is an urgent need for innovative approaches to health promotion as individual’s age. Successful research, education, and interventions should include the development of both qualitative and quantitative biomarkers and other tools which can measure improvements in physiological integrity throughout life. Data-driven health policy shifts should be aimed at reducing the socio-economic inequalities that lead to premature ageing. A framework for progress has been proposed and published by the World Health Organization in its Global Strategy and Action Plan on Ageing and Health. This symposium focused on the impact of nutrition on this framework, stressing the need to better understand an individual’s balance of intrinsic capacity and functional abilities at various life stages, and the impact this balance has on their mental and physical health in the environments they inhabit.