New chain lube claims to save seven watts and last 1800km per application.
Category: life extension
Chimeric antigen receptor (CAR) T cells have transformed the treatment of refractory blood cancers. These genetically engineered immune cells seek out and destroy cancer cells with precision. Now, scientists at Memorial Sloan Kettering are deploying them against other diseases, including those caused by senescence, a chronic “alarm state” in tissues. The scope of such ailments is vast and includes debilitating conditions, such as fibrotic liver disease, atherosclerosis, and diabetes.
Key to the success of CAR T cell therapy has been finding a good target. The first US Food and Drug Administration-approved CAR T cells target a molecule on the surface of blood cancers called CD19. It is present on cancer cells but few other normal cells, so side effects are limited.
Taking their cue from this prior work, a team of investigators including Scott Lowe, Chair of the Cancer Biology and Genetics Program in the Sloan Kettering Institute, and Michel Sadelain, Director of the Center for Cell Engineering at MSK, along with their trainees Corina Amor, Judith Feucht, and Josef Leibold, sought to identify a target on senescent cells. These cells no longer divide, but they actively send “help me” signals to the immune system.
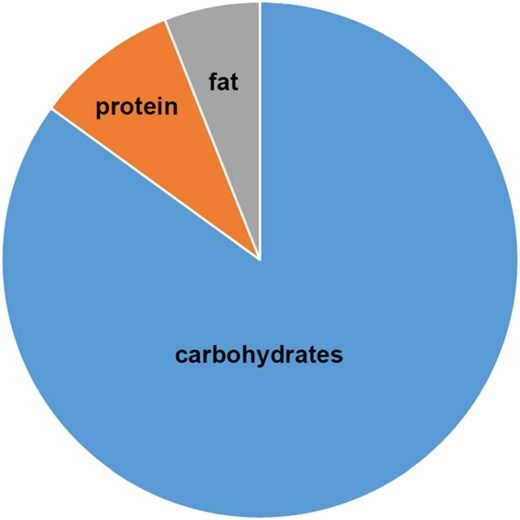
Nutrition has profound effects on ageing and lifespan. Caloric restriction is the major nutritional intervention that historically has been shown to influence lifespan and/or healthspan in many animal models. Studies have suggested that a reduction in protein intake can also increase lifespan, albeit not as dramatically as caloric restriction. More recent research based on nutritional geometry has attempted to define the effects of nutrition on ageing over a broad landscape of dietary macronutrients and energy content. Such studies in insects and mice indicate that animals with ad libitum access to low-protein, high-carbohydrate diets have longest lifespans. Remarkably, the optimum content and ratio of dietary protein to carbohydrates for ageing in experimental animals are almost identical to those in the traditional diets of the long-lived people on the island of Okinawa.
Just as our human relationships and connections can nudge, push, or dramatically shift societal values and consequences, the connections between neurons form intricate networks that dictate the outcome of your mind. Your thoughts, memories, behaviors; your values, world view, mental health—everything that makes you you is calculated and stored in these connections, called synapses, that dot our brains like billions of stars in the night sky.
If a connectome—a large-scale snapshot of all your neural connections—is a loose “copy” of you at one moment in time, synapses are a fluid representation of how you change and grow through time. Similar to human connections, synapses come in different varieties and evolve as we age. Yet until now, capturing how these synapses change as we move through time has been nearly impossible.
Last week, in a technological tour-de-force, a European team from the United Kingdom, France, and Sweden, led by Dr. Seth G.N. Grant at the University of Edinburgh, redefined impossibility with a paper in Science. Peering into the brains of mice at different ages—one day, one week, and all the way up to an elderly 18 months—the team constructed maps of roughly 5 billion synapses, outlining a timeline of their diversity and numbers in over 100 different brain regions with age.
Back in 2005, Drs. Irina and Michael Conboy showed that joining the circulatory systems of young and old mice together in a procedure called parabiosis could rejuvenate aged tissues and reverse some aspects of aging in old mice.
Following this discovery, many researchers concluded that there must be something special in young blood that was able to spur rejuvenation in aged animals, and various companies have been trying to find out what. Indeed, we recently reported that researchers were apparently successful in halving the epigenetic age of old rats by treating them with Elixir, a proprietary mix of pro-youthful factors normally found in young blood.
However, a question still remains: was the rejuvenation the result of there being something beneficial in the young blood, or is it more a case of dilution of the harmful factors present in old blood?
A pair of researchers at University College London has found that people with low socioeconomic status experience more declines in age-related functions as they grow older than do people who have a higher socioeconomic status. In their paper published in Proceedings of the National Academy of Sciences, Andrew Steptoe and Paola Zaninotto describe their study of data from the English Longitudinal Study of Ageing, and what they learned.
Prior research has shown that poor people tend to suffer more adverse health effects than those who are better off. They also tend to die younger. But one area of aging that has not been well-studied is the impact of poverty on age-related functional decline, associated with such symptoms as loss of hearing or muscle strength. To learn more about the relationship between socioeconomic status and age-related functional decline, the researchers analyzed data in the English Longitudinal Study of Ageing—an ongoing long-term study of the aging process. Launched in 2002, the study involved collecting data on volunteers aged 50 and over as they grew older. The data includes both medical and physical information, along with test results designed to measure cognitive and emotional levels. The data sample for this new effort included information on 5,018 people 52 years of age or older as they aged over periods of six to eight years.
The researchers found that people living at the lower end of the economic spectrum performed worse on every measure of age-related functionality. Those less well-off lost grip strength, lung function, gait speed, processing speed and executive function. They also tended to report enjoying life less than those who were more affluent. The researchers noted their findings were independent of race, gender, education or age. They also found that those of lesser means experienced more vision problems and were more likely to be depressed.
In 2005, University of California, Berkeley, researchers made the surprising discovery that making conjoined twins out of young and old mice—such that they share blood and organs—can rejuvenate tissues and reverse the signs of aging in the old mice. The finding sparked a flurry of research into whether a youngster’s blood might contain special proteins or molecules that could serve as a “fountain of youth” for mice and humans alike.
But a new study by the same team shows that similar age-reversing effects can be achieved by simply diluting the blood plasma of old mice—no young blood needed.
In the study, the team found that replacing half of the blood plasma of old mice with a mixture of saline and albumin—where the albumin simply replaces protein that was lost when the original blood plasma was removed—has the same or stronger rejuvenation effects on the brain, liver and muscle than pairing with young mice or young blood exchange. Performing the same procedure on young mice had no detrimental effects on their health.
Why does this happen?
To put things as simply as possible, the root cause of all aging is a loss of energy on the cellular level, and there are basically two major theories for why this occurs. One says cellular energy decline is the result of accumulated cellular and mitochondrial damage. In other words, it’s the result of wear and tear on a cellular level. The other theory speculates that it is the result of genetic programming, with some genes getting overexpressed while others get underexpressed as we age.
These two theories of cellular energy decline aren’t in competition with one another. They just look at the problem from two different vantage points. The reality is these “causes” are interrelated. Gene overexpression and underexpression can cause cellular damage. Cellular damage can impair gene expressions.
Good talk, not just about NAD. Q&A just before 35 minutes. A lot of epigenetics here.
David A. Sinclair, Ph.D., A.O. is a Professor in the Department of Genetics and co-Director of the Paul F. Glenn Center for the Biology of Aging at Harvard Medical School. He is best known for his work on understanding why we age and how to slow its effects. He obtained his Ph.D. in Molecular Genetics at the University of New South Wales, Sydney in 1995. He worked as a postdoctoral researcher at M.I.T. with Dr. Leonard Guarente where he co discovered a cause of aging for yeast as well as the role of Sir2 in epigenetic changes driven by genome instability. In 1999 he was recruited to Harvard Medical School where he has been teaching aging biology and translational medicine for aging for the past 16 years. His research has been primarily focused on the sirtuins, protein-modifying enzymes that respond to changing NAD+ levels and to caloric restriction (CR) with associated interests in chromatin, energy metabolism, mitochondria, learning and memory, neurodegeneration, and cancer. The Sinclair lab was the first one to identify a role for NAD+ biosynthesis in regulation of lifespan and first showed that sirtuins are involved in CR in mammals. They first identified small molecules that activate SIRT1 such as resveratrol and studied how they improve metabolic function using a combination of genetic, enzymological, biophysical and pharmacological approaches. They recently showed that natural and synthetic activators require SIRT1 to mediate the in vivo effects in muscle and identified a structured activation domain. They demonstrated that miscommunication between the mitochondrial and nuclear genomes is a cause of age-related physiological decline and that relocalization of chromatin factors in response to DNA breaks may be a cause of aging.
DARPA, Biotech, and Human Enhancement — ideaXme — Dr. Eric Van Gieson — Biological Technologies Office (BTO) Epigenetic CHaracterization and Observation (ECHO) Program — Ira Pastor
Posted in aging, bioengineering, biotech/medical, defense, DNA, genetics, government, health, life extension, military | Leave a Comment on DARPA, Biotech, and Human Enhancement — ideaXme — Dr. Eric Van Gieson — Biological Technologies Office (BTO) Epigenetic CHaracterization and Observation (ECHO) Program — Ira Pastor