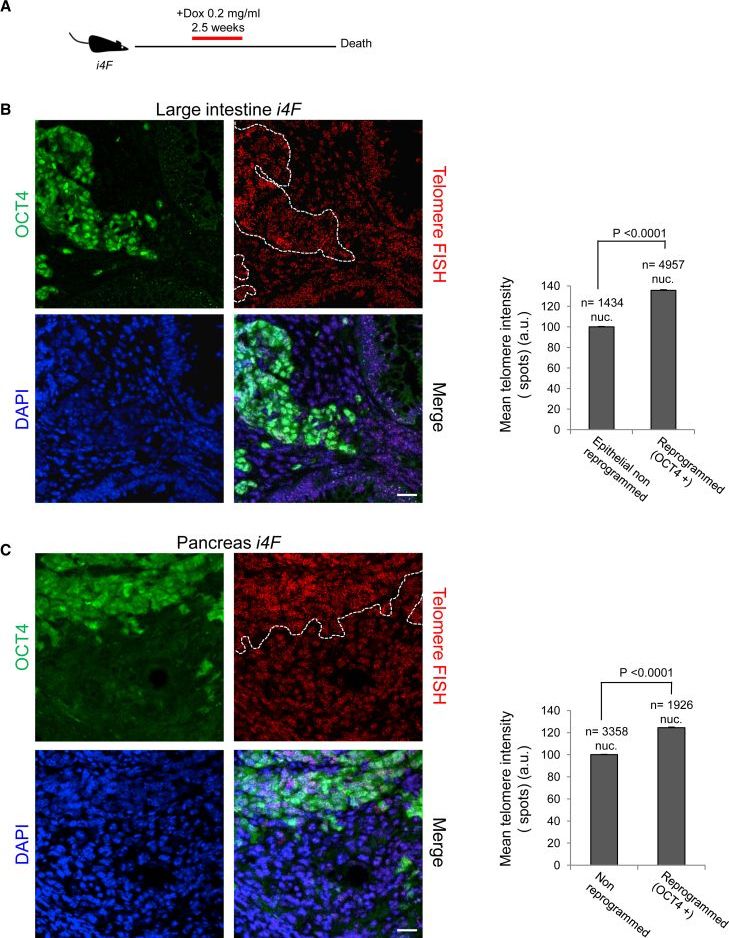
Reprogramming of differentiated cells into induced pluripotent stem cells has been recently achieved in vivo in mice. Telomeres are essential for chromosomal stability and determine organismal life span as well as cancer growth. Here, we study whether tissue dedifferentiation induced by in vivo reprogramming involves changes at telomeres. We find telomerase-dependent telomere elongation in the reprogrammed areas. Notably, we found highly upregulated expression of the TRF1 telomere protein in the reprogrammed areas, which was independent of telomere length. Moreover, TRF1 inhibition reduced in vivo reprogramming efficiency. Importantly, we extend the finding of TRF1 upregulation to pathological tissue dedifferentiation associated with neoplasias, in particular during pancreatic acinar-to-ductal metaplasia, a process that involves transdifferentiation of adult acinar cells into ductal-like cells due to K–Ras oncogene expression. These findings place telomeres as important players in cellular plasticity both during in vivo reprogramming and in pathological conditions associated with increased plasticity, such as cancer.
Keywords: in vivo reprogramming, telomeres, stem cells, TRF1, tumorigenesis, cellular plasticity, cancer, transdifferentiation, ADM, regeneration.
Reprogramming into full pluripotency has been achieved in vivo in the context of mouse tissues (Abad et al., 2013). Thus, induction of the reprogramming factors in transgenic mice (so-called reprogrammable mice) results in reprogramming events marked by the expression of the pluripotency factor NANOG in multiple organs, tissue dedifferentiation, and teratoma formation. Therefore, these mice could be useful for a deeper understanding of the molecular mechanisms that govern tissue dedifferentiation in vivo. Interestingly, mammalian cell reprogramming can also occur spontaneously during regeneration after injury or damage conditions (Yanger et al., 2013). Differentiated cells can be converted in vivo into another cell type and also into functional multipotent stem-like cells (Tata et al., 2013). This capacity of somatic cells to dedifferentiate into stem-like cells in vivo may have a pivotal role in physiological tissue regeneration or during tumorigenesis.