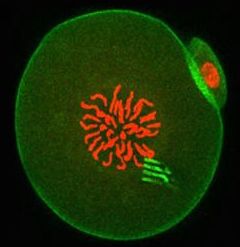
For the first time, scientists have introduced minuscule tracking devices directly into the interior of mammalian cells, giving an unprecedented peek into the processes that govern the beginning of development. This work on one-cell embryos is set to shift our understanding of the mechanisms that underpin cellular behaviour in general, and may ultimately provide insights into what goes wrong in ageing and disease.
The research, led by Professor Tony Perry from the Department of Biology and Biochemistry at the University of Bath, involved injecting a silicon-based nanodevice together with sperm into the egg cell of a mouse. The result was a healthy, fertilised egg containing a tracking device.
The tiny devices are a little like spiders, complete with eight highly flexible ‘legs’. The legs measure the ‘pulling and pushing’ forces exerted in the cell interior to a very high level of precision, thereby revealing the cellular forces at play and showing how intracellular matter rearranged itself over time.