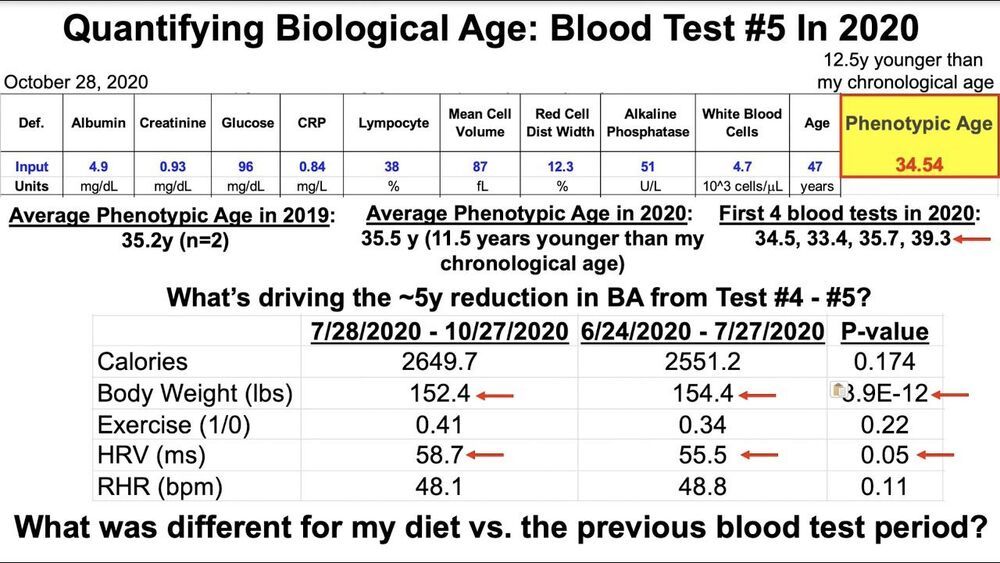
Hi everybody. Today, it was published a paper in which it’s described the research that led to the identification and testing of a peptide that reduces the amount of senescent cells in the skin, and that peptide is being used in the first product in the whole world (as far as I know) that is already in the market and reduces the amount of senescent cells in humans (in this case, in the skin). The paper can be found in I don’t think it’s an ordinary thing that a product that reduces the amount of senescent cells is being sold in the market. After many years watching Aubrey de Grey’s talks, and reading news about promising researches about senescent cells, and about the formation of many companies to research how to reduce the amount of senescent cells, finally there is something that reached the public. This paper is very important as it allows that the rejuvenation field analyzes it and be prepared to seize this opportunity to show to the world, in practice, that the theoretical base of the rejuvenation therapies can be translated to practice and rejuvenate the human body — in this case, the skin. As some of you already know, the company which organized this research and launched the product is OneSkin, and its CEO, Carolina Reis, has been presenting their research in several conferences in the rejuvenation field in the last months. OneSkin is a company with its interests, of course, but at the same time is a representative of the rejuvenation field which is opening a path for all the other companies.
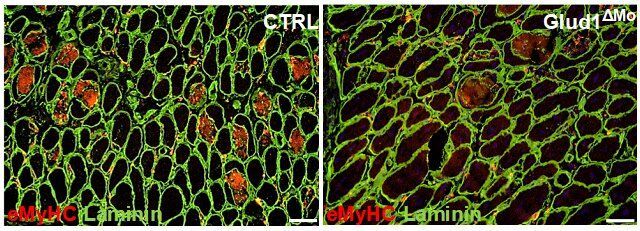
Skin aging has been primarily related to aesthetics and beauty. Therefore, interventions have focused on reestablishing skin appearance, but not necessarily skin health, function, and resilience. Recently, cellular senescence was shown to play a role in age-related skin function deterioration and influence organismal health and, potentially, longevity. In the present study, a two-step screening was performed to identify peptides capable of reducing cellular senescence in human dermal fibroblasts (HDF) from Hutchinson-Gilford Progeria (HGPS) patients. From the top four peptides of the first round of screening, we built a 764-peptide library using amino acid scanning, of which the second screen led to the identification of peptide 14. Peptide 14 effectively decreased HDF senescence induced by HGPS, chronological aging, ultraviolet-B radiation, and etoposide treatment, without inducing significant cell death, and likely by modulating longevity and senescence pathways. We further validated the effectiveness of peptide 14 using human skin equivalents and skin biopsies, where peptide 14 promoted skin health and reduced senescent cell markers, as well as the biological age of samples, according to the Skin-Specific DNA methylation clock, MolClock. Topical application of peptide 14 outperformed Retinol treatment, the current gold-standard in anti-aging skincare. Finally, we determined that peptide 14 is safe for long-term applications and also significantly extends both the lifespan and healthspan of C. elegans worms tested in two independent testings. This highlights the potential for geroprotective applications of the senotherapeutic compounds identified using our screening platform beyond the skin.
MB, AZ, CR, LB, EA, and JC are named as inventors of a patent directed at this invention, which is solely owned by OneSkin, Inc. MB, AZ, CR, EA, and JC are co-founders of OneSkin Inc. SAV and MR are co-founders of the startup company NemaLife Inc. that is commercializing microfluidic devices used in this study and licensed from Texas Tech University. SAV, MR, and TA are named inventors on a patent owned by Texas Tech University and receive royalty fees.