Category: life extension

Dr Rhonda Prisby: From blood to bone and the connections between cardiovascular and skeletal systems
From Blood to Bone (and back)! — Dr. Rhonda Prisby, from University of Texas at Arlington, joins me on ideaXme (http://radioideaxme.com/) to discuss her fascinating research in the Bone Vascular and Micro-Circulation Laboratory, focusing on the unique interaction between vascular and skeletal systems, and novel disease states where vessels become bone-like “dead space”! — https://www.youtube.com/watch?v=PsK-pPjW020&t=1s #Ideaxme #Bone #Microcirculation #Vasculature #Ossification #Atherosclerosis #Parathyroid #Osteoblast #Osteoclast #Health #Wellness #Regeneration #Longevity #Aging #IraPastor #Bioquark #Regenerage
Ira Pastor, ideaXme exponential health ambassador, interviews Dr. Rhonda Prisby, Associate Professor in the Department of Kinesiology, at The University of Texas at Arlington.
Ira Pastor comments:
We have a fascinating show today focusing on the intersection of the cardiovascular system and the skeletal system.
We will be discussing interesting recent evidence that suggests a unique link between cardiovascular disease and osteoporosis, a disease in which both the density and quality of bone are reduced, where bones become more porous and fragile, and the risk of fracture is greatly increased.
Bone Blood Vessels:
It has been known that adequate vascular supply is critical for proper bone healing following fracture, and that bone blood vessels participate in a variety of physiological processes in the bone marrow.
However, little is known about how the blood vessels in bone contribute to bone health and disease, as well as the reverse – that is how all of the unique dynamics in the bone micro-environment have effects on other tissues.

LifeXtenShow – Will Life Extension Kill the Pension System?
Giuliano uses this LE 101 episode of LifeXtenShow to explain what might happen to the pension system if partial, or total, rejuvenation comes to pass – and discusses why such a system was created in the first place.
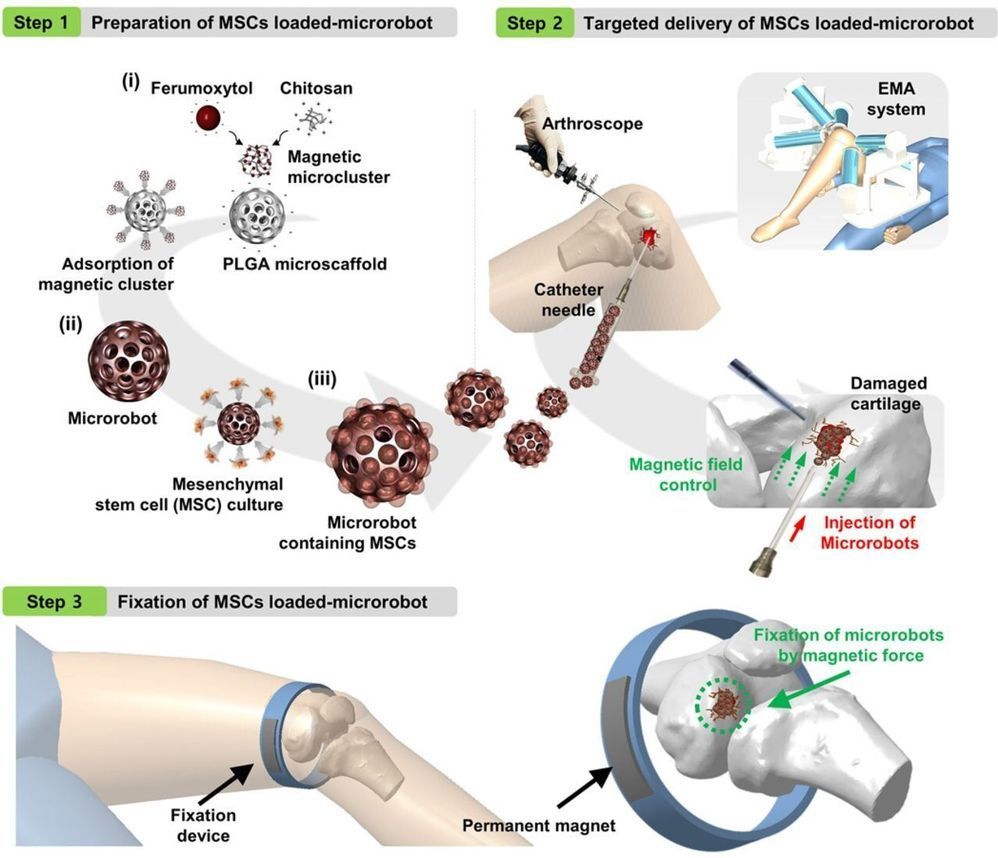
Microrobot system regenerates knee cartilage in rabbits
A team of researchers affiliated with multiple institutions in China and one in Korea has developed a micro-robot system that regenerated knee cartilage in rabbits. In their paper published in the journal Science Advances, the group describes their system and how well it worked.
In many developed countries, the population is growing older, which means aging-related health conditions are on the rise. One such ailment common in older people is degeneration of the cartilage in the knees and hips. When this happens, a common treatment is replacing the knee or hip joint with an artificial device. In this new effort, the researchers have found a better way to handle the problem—regrowing the cartilage.
Prior research has shown that mesenchymal stem cells found in bone marrow and fat can be coaxed into growing into cartilage cells. And researchers have also found that stem cells can be used to repair damaged cartilage. The challenge is placing the cells in the body where they are needed and keeping them in place until they attach to the surrounding tissue. In this new effort, the researchers have created a system that was able to overcome these hurdles—at least in rabbits.
Researchers can reprogramme cells to original state for regenerative medicine
Early mammalian development is a highly complex process involving elaborate and highly coordinated biological processes. One such process is zygotic genome activation (ZGA) which occurs following the union of the sperm and egg, marking the beginning of life. The resultant early embryos, termed ‘zygotes’ are capable of generating the entire organism, a property known as totipotency.
Totipotent cells sit atop the developmental hierarchy and have the greatest potency of all cell types, giving it limitless therapeutic potential. Surpassing pluripotent embryonic stem cells, which are only able to differentiate into all cell types within the embryo, the totipotent zygote loses its totipotency as it matures into pluripotency.
Scientists at the National University of Singapore’s Yong Loo Lin School of Medicine have now found a way to manipulate pluripotent cells into acquiring the totipotent capacity previously thought to exist only in the zygote. This not only provides key insights into how totipotency is formed and the earliest events in mammalian development, but opens new doors for potential cell therapies that were previously unexplored.

Building an Atlas of Senescent Cell Secretions
Recently, a team of researchers, including Professor Judy Campisi, has published an atlas charting the inflammatory senescence-associated secretory phenotype (SASP) [1].
The nature of the SASP
As we grow older, increasing numbers of our cells enter a state known as senescence. Senescent cells no longer divide to support and help maintain the tissues that they are a part of and instead secrete a range of harmful inflammatory signals: the SASP.

Brian Kennedy Joins the LEAF Scientific Advisory Board
 We are delighted to announce that Dr. Brian Kennedy, a Distinguished Professor in the Department of Biochemistry and Physiology at the National University of Singapore (NUS) will be joining the LEAF scientific advisory board.
We are delighted to announce that Dr. Brian Kennedy, a Distinguished Professor in the Department of Biochemistry and Physiology at the National University of Singapore (NUS) will be joining the LEAF scientific advisory board.
Professor Kennedy is an important figure in the research community, as he is internationally recognized for his research and efforts to translate those findings into therapies that could potentially slow, delay, or even prevent age-related diseases. He previously served as the President of the Buck Institute, where he still remains as a Professor.
At the NUS, he is developing therapeutic interventions that directly target human aging along with biomarkers that can validate if a therapy has worked or not. Professor Kennedy and his team have been exploring the epigenetic clock, a biomarker that measures methylation of the human genome to determine biological age. They are also investigating inflammatory biomarkers of aging using metabolomics, the study of chemical processes involving metabolites, the intermediates and products of metabolism.

This month we will be taking a look at the results of a recent human trial where the drug rapamycin was used to treat skin aging with some promising results
Link to study paper: https://link.springer.com/…/10.1007%2Fs11357-019-00113-y.pdf

Ways to Live Forever
https://www.youtube.com/watch?v=kPhJ20XubtM&feature=share