New research exploring theories of aging has found that small mutations accumulating in DNA are unlikely to be fully responsible for this process.
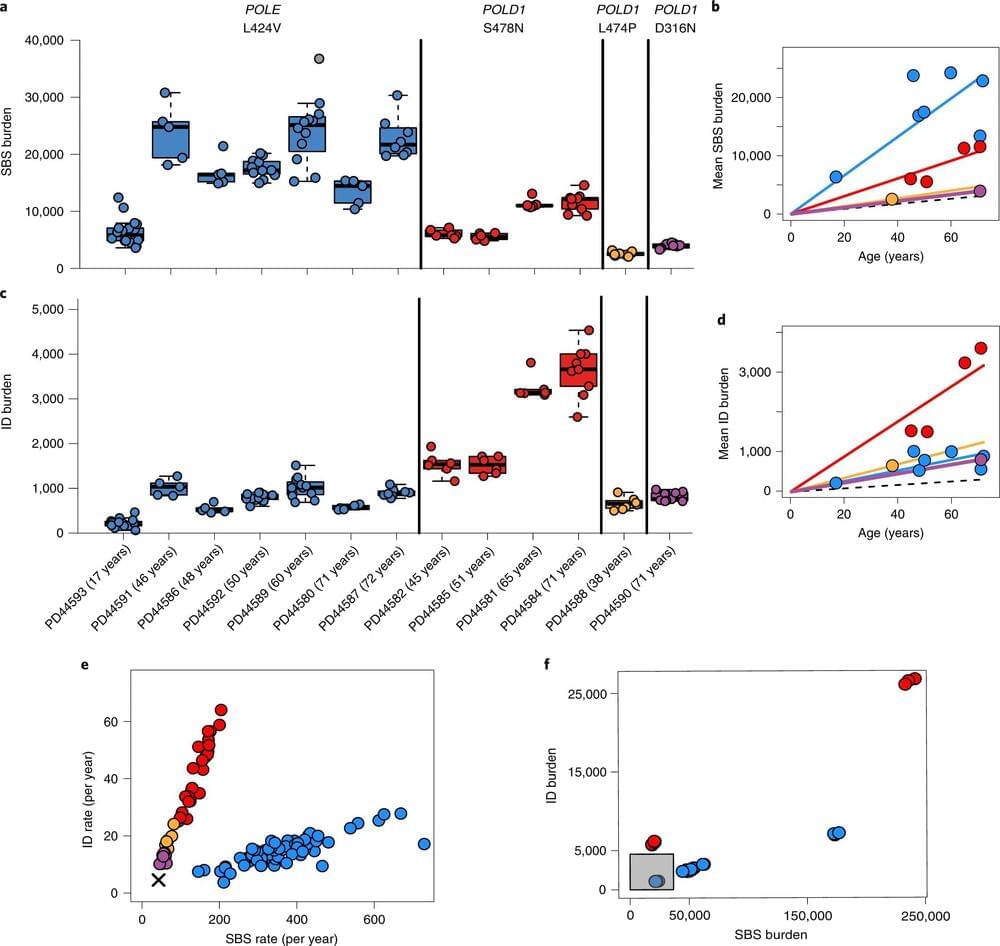
The research, a collaboration between the Wellcome Sanger Institute, University of Birmingham, University of Edinburgh and others, found that human cells and tissues can accumulate many more mutations than are normally present, without the body showing the features associated with aging.
The new study, published today (30 September) in Nature Genetics, compared DNA taken from individuals with inherited mutations in genes involved in DNA replication with DNA from individuals who have normal versions of these genes. The researchers aimed to understand the impact of defective DNA replication on cancer risk and features associated with aging. The results suggest that build-up of mutations in normal cells is unlikely to be the only factor in the development of age-related disease, adding to the ongoing debate about the causes of aging.