A company formed by Harvard genetics professor George Church, known for his pioneering work in genome sequencing and gene splicing, hopes to genetically resurrect woolly mammoths.



Because the herpesvirus sits in neurons forever, there is speculation it is connected to neurodegenerative diseases. The immune system requires inflammation to constantly fight off the virus, and neurons have some degree of damage because of this continuous immune response, according to Dr. Tibor Valyi-Nagy, professor of pathology, director of neuropathology at UIC and research collaborator on the study.
Summary: Researchers discovered mutations of the OPTN gene resulted in increased herpesvirus 1 growth in the brains of mice, leading to the death of local neurons. This resulted in accelerated neurodegeneration. OPTN deficiency was also associated with impairments in immune response. While these findings are specific to the HSV-1 virus, researchers believe the findings may apply to up to eight herpesvirus infections.
Source: University of Illinois at Chicago
A new study by researchers at University of Illinois Chicago suggests that when the protein optineurin, or OPTN, is present in cells it restricts the spread of HSV-1, the herpes simplex virus type 1.
In a “first of its kind” study, researchers also found a potential direct connection between neurodegenerative diseases, such as Alzheimer’s disease, amyotrophic lateral sclerosis (ALS), glaucoma, and the herpesvirus, said Dr. Deepak Shukla, the Marion H. Schenk Esq. Professor in Ophthalmology for Research of the Aging Eye, and vice chair for research at UIC.

As we age, our bones become thinner, we suffer fractures more often, and bone-diseases such as osteoporosis are more likely to occur. One responsible mechanism involves the impaired function of the bone-marrow stem cells, which are required for the maintenance of bone integrity. Researchers from the Max Planck Institute for Biology of Ageing and CECAD Cluster of Excellence for Ageing Research at the University of Cologne have now shown that the reduced stem cell function upon aging is due to changes in their epigenome. They were able to reverse these changes in isolated stem cells by adding acetate. This fountain of youth for the epigenome could become important for the treatment of diseases such as osteoporosis.
Aging Researchers have been looking at epigenetics as a cause of aging processes for some time. Epigenetics looks at changes in genetic information and chromosomes that do not alter the sequence of the genes themselves, but do affect their activity. One possibility is changes in proteins called histones, which package the DNA in our cells and thus control access to DNA. The Cologne research group of Peter Tessarz has now studied the epigenome of mesenchymal stem cells. These stem cells are found in bone marrow and can give rise to different types of cells such as cartilage, bone and fat cells.
This is an emerging therapeutic technique that works by targeting the underlying causes of a disease, rather than the symptoms it causes. It does this by targeting a particular gene, and preventing it from making the protein that it produces.
The United Kingdom’s NHS has very recently approved a new cholesterol-lowering jab which will be offered to 300,000 people over the next three years.
The drug – inclisiran – will be administered twice a year as an injection.
It will mainly be prescribed to patients who suffer with a genetic condition that leads to high cholesterol, those who have already suffered a heart attack or stroke, or those who haven’t responded well to other cholesterol-lowering drugs, such as statins.
Within the last decade, scientists have adapted CRISPR systems from microbes into gene editing technology, a precise and programmable system for modifying DNA. Now, scientists at MIT’s McGovern Institute and the Broad Institute of MIT and Harvard have discovered a new class of programmable DNA modifying systems called OMEGAs (Obligate Mobile Element Guided Activity), which may naturally be involved in shuffling small bits of DNA throughout bacterial genomes.
These ancient DNA-cutting enzymes are guided to their targets by small pieces of RNA. While they originated in bacteria, they have now been engineered to work in human cells, suggesting they could be useful in the development of gene editing therapies, particularly as they are small (~30% the size of Cas9), making them easier to deliver to cells than bulkier enzymes. The discovery, reported in the journal Science, provides evidence that natural RNA-guided enzymes are among the most abundant proteins on earth, pointing toward a vast new area of biology that is poised to drive the next revolution in genome editing technology.
The research was led by McGovern investigator Feng Zhang, who is James and Patricia Poitras Professor of Neuroscience at MIT, a Howard Hughes Medical Institute investigator, and a core institute member of the Broad Institute. Zhang’s team has been exploring natural diversity in search of new molecular systems that can be rationally programmed.

Circa 2014
New collaborative research published in the journal Nature Communications by scientists from Japan, Russia and the US contains the genetic analysis on a species of African midge, which can survive a wide array of extreme conditions including large variations in temperature, extreme drought and even airless vacuums such as space. The team successfully deciphered the genetic mechanism that makes the midge invulnerable to these harsh conditions. Prof. Noriyuki Satoh and Dr. Takeshi Kawashima of Prof. Satoh’s Marine Genomics Unit, as well as Prof. Alexander Mikeyhev of the Ecology and Evolution Unit, and Mr. Manabu Fujie and Dr. Ryo Koyanagi of the DNA Sequencing Section at the Okinawa Institute of Science and Technology Graduate University have contributed to the collaboration.
The midge, Polypedilum vanderplanki, is capable of anhydrobiosis, a unique state that allows an organism to survive even after losing 97% of its body water. Anhydrobiotic organisms are also able to survive other severe conditions such as extreme temperatures ranging from 90°C to-270°C, vacuums and high doses of radiation; all of which would be lethal to most other life forms.
The midge found in northern Nigeria lives in an environment where the dry season lasts for at least six months and droughts can last up to eight months. By the time eggs have hatched and larvae have developed, the pools of water they breed in have dried up. However these dried larvae can survive in this dehydrated state for more than 17 years. “This is a very interesting kind of phenomena,” remarks Prof. Satoh. “The first descriptions of this midge were more than 60 years ago… But serious research started only ten years ago.”
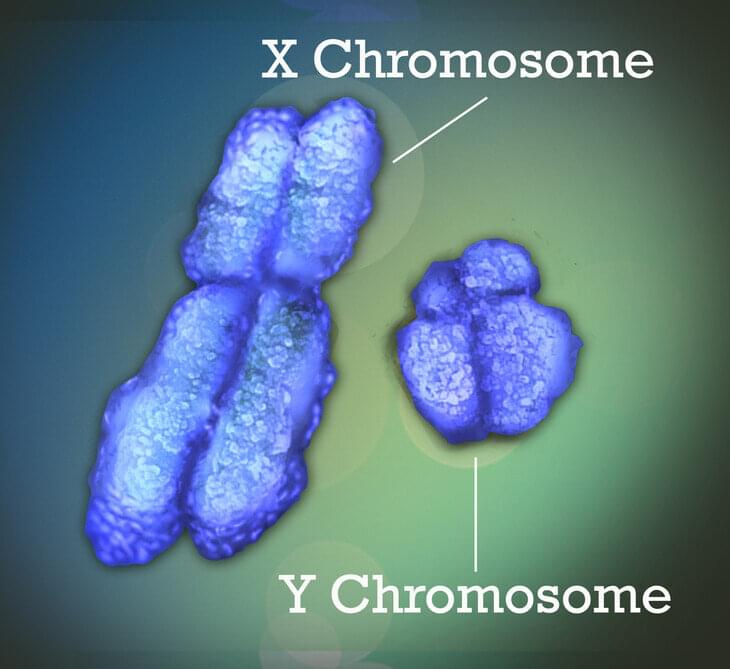
Many human diseases can differ between males and females in their prevalence, manifestation, severity or age of onset. Examples include Lupus, where more than 80% of patients are females; Alzheimer’s disease, where females have higher incidence and tend to suffer quicker cognitive decline; and COVID-19 infections that are frequently more severe in males.
These sex differences may have a genetic basis that is attributable to the sex chromosomes. The X chromosome—one of the two sex chromosomes—is known to play an important role in human development and disease. New research led by Penn State College of Medicine reveals for the first time that sex-biased diseases can be attributable to genes that escape X chromosome inactivation (XCI), a process that ensures that females do not overexpress genes on their X-chromosomes.
The team developed a genetic tool that can identify these XCI escape genes, and it may also help in determining whether a female will develop a sex-biased disease and if the disease will become progressively worse over time. The tool may even be useful in understanding the sex differences in immune responses to COVID-19, as the disease is thought to produce more severe symptoms and higher mortality in men than in women.

Circa 2000
A 1940 paper by Gamow and Mario Schoenberg was the first in a subject we now call particle astrophysics. The two authors presciently speculated that neutrinos could play a role in the cooling of massive collapsing stars. They named the neutrino reaction the Urca process, after a well known Rio de Janeiro casino. This name might seem a strange choice, but not to Gamow, a legendary prankster who once submitted a paper to Nature in which he suggested that the Coriolis force might account for his observation that cows chewed clockwise in the Northern Hemisphere and counterclockwise in the Southern Hemisphere.
In the 1940s Gamow began to attack, with his colleague Ralph Alpher, the problem of the origin of the chemical elements. Their first paper on the subject appeared in a 1948 issue of the Physical Review. At the last minute Gamow, liking the sound of ‘alpha, beta, gamma’, added his old friend Hans Bethe as middle author in absentia (Bethe went along with the joke, but the editors did not). Gamow and Alpher, with Robert Herman, then pursued the idea of an extremely hot neutron-dominated environment. They envisioned the neutrons decaying into protons, electrons and anti-neutrinos and, when the universe had cooled sufficiently, the neutrons and protons assembling heavier nuclei. They even estimated the photon background that would be necessary to account for nuclear abundances, suggesting a residual five-degree background radiation.
We now realize that their scheme was incorrect. The Universe began with roughly equal numbers of protons and neutrons. Collisions with electrons, positrons, neutrinos and anti-neutrinos are more important than neutron decay, and the absence of stable nuclei with atomic numbers of five and eight creates a barrier to further fabrication in the early Universe. Nevertheless Alpher, Gamow and Herman’s work was the first serious attempt to discuss the observable consequences of a big bang and the basic framework was correct. Ironically, the term ‘Big Bang’ was coined by Fred Hoyle, an advocate of a steady-state model of the universe, to make fun of Gamow’s efforts.
Mentions telomeres.
~~~
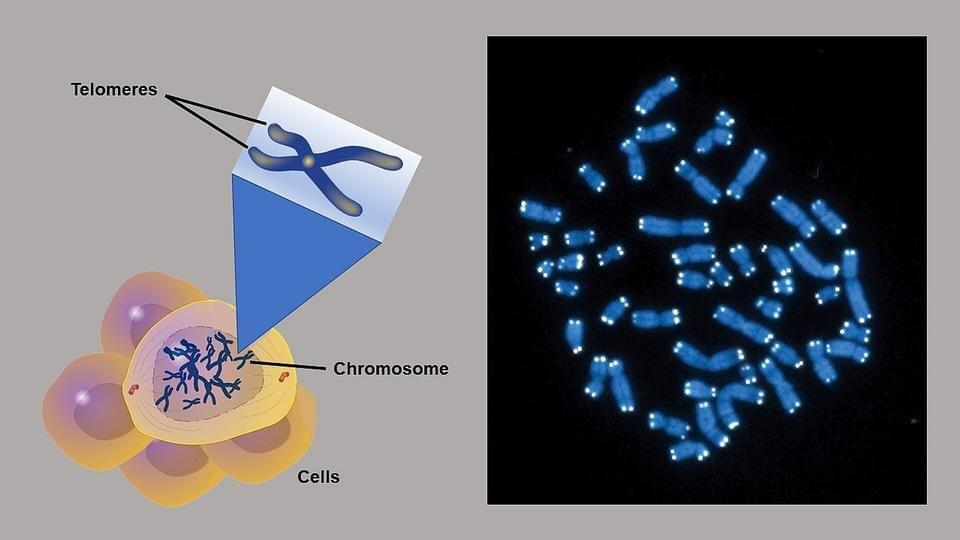
People with rare disorders that cause shortened telomeres—protective caps that sit at the end of chromosomes—may be more likely to have blood cancers such as leukemia or myelodyplastic syndrome. Now, Johns Hopkins Medicine scientists have discovered several “self-correcting” genetic mutations in bone marrow that may protect such patients from these cancers.
In a study published online August 3 2021, in the Journal of Clinical Investigation, the researchers also suggest these mutations can serve as biomarkers that may indicate if patients with short telomere syndromes are likely to develop blood cancers.
“These are the most common cancers we see in patients with short telomere syndromes,” says Mary Armanios, M.D., director of the Telomere Center and professor of oncology at the Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins. “We know that at a certain point, the cells of patients with shortened telomeres either become cancerous or stay healthy.”

Summary: Researchers have identified 2,000 genes in humans linked to longevity. The genes are associated with biological mechanisms that drive the prolongation of life in mammals, including DNA repair, coagulation, and immune response.
Source: UPF Barcelona.
What determines the life expectancy of each species? This is a fundamental and highly complex question that has intrigued the field of research throughout history. From the evolutionary point of view, the major cause of these differences between species lies in their ecological adaptations. For example, life expectancy is longer in species adapted to living in trees, underground, or with large body mass, since all these adaptations reduce mortality by predation.