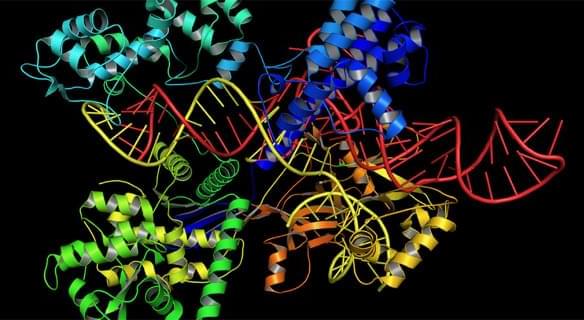
A model of facilitated diffusion and the theory of Anderson localization help explain how the Cas9 protein explores DNA in search of its targets.
Category: biotech/medical
To understand why Mark Zuckerberg thinks “the metaverse” is the next frontier, consider the case of Sam Peurifoy. The 27-year-old chemistry PhD from Columbia University left his job at Goldman Sachs at the height of the pandemic and is now seeking out his fortune in crypto by playing video games.
He has recruited dozens of people from Mexico to the Philippines to a “Guild” that plays under the command of “Captain” Peurifoy. In exchange, he ponies up the funds needed to enter Axie Infinity, a game where players collect Smooth Love Potion — a digital token that can potentially be converted into real money.
Aging affects everybody, so it’s easy to understand why so much scientific attention is focused on studying it. Scientists in Canada now claim to have found that telomeres play a different role in cellular aging than previously thought.
One of the main points of interest in anti-aging biology are what’s known as telomeres. These are sections of “junk” DNA that form caps on the ends of chromosomes, protecting important genetic information from damage when a cell divides. But a piece of the telomere is eroded away with each cell division, and when it gets too short the cell stops dividing entirely, entering a dormant state known as senescence. The build-up of these senescent cells contributes to a range of symptoms we associate with aging, such as frailty and age-related diseases.
The implication of this model is that telomeres take on a pre-emptive protection role – they signal to cells to stop dividing as soon as one telomere wears out. But there is evidence to suggest that cell division can continue with as many as five dysfunctional telomeres.
The problem? Our bodies aren’t big fans of foreign substances—particularly ones that trigger an undesirable immune response. What’s more, these delivery systems aren’t great with biological zip codes, often swarming the entire body instead of focusing on the treatment area. These “delivery problems” are half the battle for effective genetic medicine with few side effects.
“The biomedical community has been developing powerful molecular therapeutics, but delivering them to cells in a precise and efficient way is challenging,” said Zhang at the Broad Institute, the McGovern Institute, and MIT.
Enter SEND. The new delivery platform, described in Science, dazzles with its sheer ingenuity. Rather than relying on foreign carriers, SEND (selective e ndogenous e n capsidation for cellular delivery) commandeers human proteins to make delivery vehicles that shuttle in new genetic elements. In a series of tests, the team embedded RNA cargo and CRISPR components inside cultured cells in a dish. The cells, acting as packing factories, used human proteins to encapsulate the genetic material, forming tiny balloon-like vessels that can be collected as a treatment.
Yekaterina “Kate” Shulgina was a first year student in the Graduate School of Arts and Sciences, looking for a short computational biology project so she could check the requirement off her program in systems biology. She wondered how genetic code, once thought to be universal, could evolve and change.
That was 2016 and today Shulgina has come out the other end of that short-term project with a way to decipher this genetic mystery. She describes it in a new paper in the journal eLife with Harvard biologist Sean Eddy.
The report details a new computer program that can read the genome sequence of any organism and then determine its genetic code. The program, called Codetta, has the potential to help scientists expand their understanding of how the genetic code evolves and correctly interpret the genetic code of newly sequenced organisms.
Designing Plants To Bring Quality Of Life — Dr. Björn Örvar, Ph.D., CSO, EVP, Co-Founder, ORF Genetics (Iceland)
Dr. Björn Lárus Örvar, Ph.D. is Chief Scientific Officer, Executive VP of Business Development, and a Co-Founder of ORF Genetics (https://www.orfgenetics.com/), an innovative plant biotechnology company and a pioneer in developing and manufacturing high-quality recombinant proteins, such as growth factors, derived from barley plants.
ORF Genetics was established in 2001 to develop innovative, economically viable and enabling solutions to produce recombinant proteins, using barley grain as a vehicle for their production, providing a more efficient and safer method than other protein expression systems provide.
Dr. Örvar served as the CEO of the company from 2006 to 2,013 and the Executive V.P. and Chief Scientific Officer of ORF Genetics since 2,013 and was the Member of the Board of ORF Genetics from 2001 to 2006.
Dr. Örvar received his Ph.D. in plant molecular genetics in 1997 from the University of British Columbia, Canada, and was a post-doctoral fellow at McGill University, Montréal for three years, focusing on plant cell stress signalling.
Dr. Örvar is responsible for research and innovation within the company as well as being the international spokesperson for ORFs skincare brand, BIOEFFECT.
Making science serve humanity: Jennifer Doudna, PhD, says CRISPR gene-editing technology should be accessible to all
Posted in biotech/medical, genetics, health, science | Leave a Comment on Making science serve humanity: Jennifer Doudna, PhD, says CRISPR gene-editing technology should be accessible to all
The path that led Jennifer Doudna, PhD, and her colleagues to the development of CRISPR, the gene-editing tool that has revolutionized science and earned her a Nobel Prize, started with their deep curiosity and drive to understand how the most basic building blocks of life function.
When Doudna first decided to investigate precisely what systems bacteria use to adapt their immune systems to fight off viral infections, she had little expectation that the findings would ultimately provide the key to technology that could be used to safely alter genetic code.
“All of us [on the research team] realized that what had started as a fundamental research question was morphing into a very different kind of project; namely, one with enormous technical potential and also risks and opportunities that we had not appreciated when we started the work,” Doudna explained during a conversation with J. Larry Jameson, MD, PhD, chair of the AAMC Board of Directors and executive vice president of the University of Pennsylvania Health System, at the opening plenary of Learn Serve Lead 2021: The Virtual Experience, on Monday, Nov. 8.
A CNS 2021 provided an incredible opportunity to learn more how the anatomy and integrity of brain networks impact higher-level cognition.
In the 19th and 20th century, cases of individuals with brain injury, such as Phineas Gage or Henry Molaison, have advanced our understanding of the relationship between the anatomy of the brain and its function. Back then, methods were limited to investigate whole-brain structure and function. Now, cognitive neuroscientists have some ability to visualize and measure activity of the whole brain at once, as well as the computational tools to investigate complex network-level relationships between brain structure, brain function, and behavior.
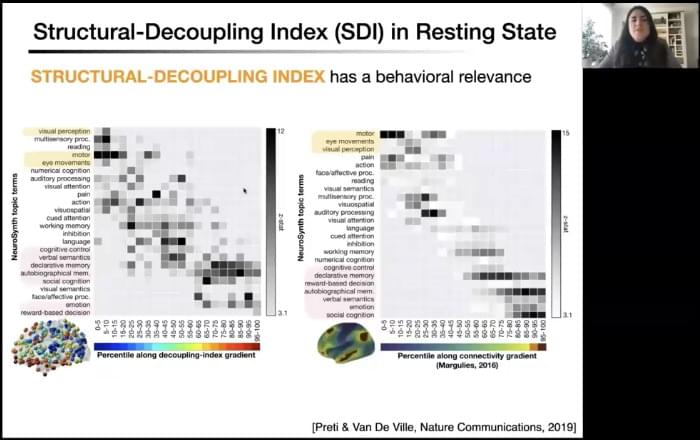
As a doctoral student working on stroke recovery, attending the CNS 2021 symposium led by Danielle Bassett was an incredible opportunity to learn more about some of the most recent methods that have been developed to understand how the anatomy and integrity of brain networks impact higher-level cognition. Strokes highly disrupt anatomical and functional connectivity, leading to cognitive and motor impairments. In individuals with post-stroke language impairments, namely aphasia, evidence shows that the more functional brain networks recover an organization similar to healthy individuals the better the recovery (Kiran et al., 2019). Understanding the relationship between brain structure and function in health and disease is therefore essential to develop appropriate treatments.
At this CNS symposium, the speakers showed how different models can help us better understand brain structural organization and how this particular organization constrains cognitive processes. They also showed direct relationships between alteration of anatomical networks, caused by disease or behavioral training, and changes in behavioral performance.
China’s zero-COVID-19 policy is showing strain as health authorities struggle to contain the growing spread of the Delta variant.
At least 1,000 locally-transmitted infections have been reported since mid-October in 20 provinces, prompting strict quarantine periods and area-specific lockdowns.
The onset of winter in China’s north is also helping disperse the disease.
Al Jazeera’s Katrina Yu reports from Beijing, China.
- Follow us on Twitter: https://twitter.com/AJEnglish/
- Find us on Facebook: https://www.facebook.com/aljazeera/
- Check our website: https://www.aljazeera.com/
#China #COVID #Delta