Senescence in cancer cells
~~~
Sometimes, too much of a good thing can turn out to be bad. This is certainly the case for the excessive cell growth found in cancer. But when cancers try to grow too fast, this excessive speed can cause a type of cellular aging that actually results in arrested growth. Scientists at Duke-NUS Medical School have now discovered that a well-known signaling pathway helps cancers grow by blocking the pro-growth signals from a second major cancer pathway.
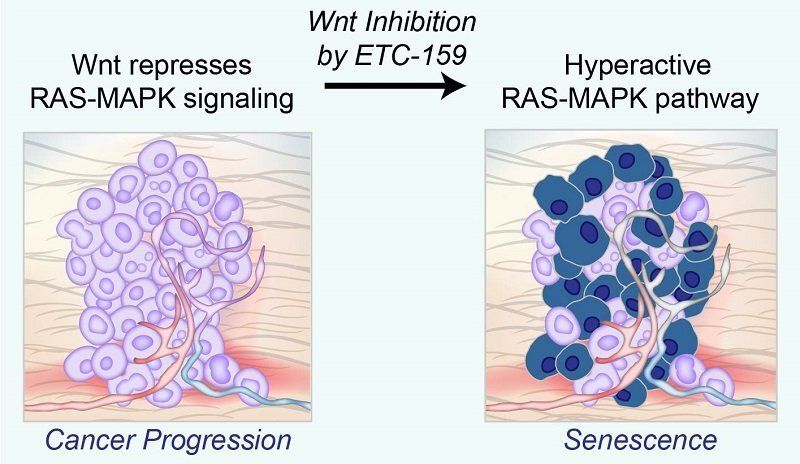
Inhibiting Wnt signaling with ETC-159 reactivates the hyperactive RAS-MAPK pathway, causing cells to led undergo senescence. Many cancers are driven by activating mutations in the RAS-MAPK signaling pathway which triggers a cascade of proteins that directs cells to grow, divide and migrate. Mutations in proteins involved in this cascade can turn on genes that make this process go into overdrive, causing cells to grow out of control and aggressively invade other parts of the body. However, too much RAS-MAPK signaling causes cancer cells to prematurely age, and eventually stop growing—a process called cellular senescence.
Publishing in Cancer Research, the Duke-NUS research team found that another important and well-known biochemical pathway, the Wnt (pronounced “wint”) signaling pathway, allows some cancers to grow by dampening RAS-MAPK signaling.