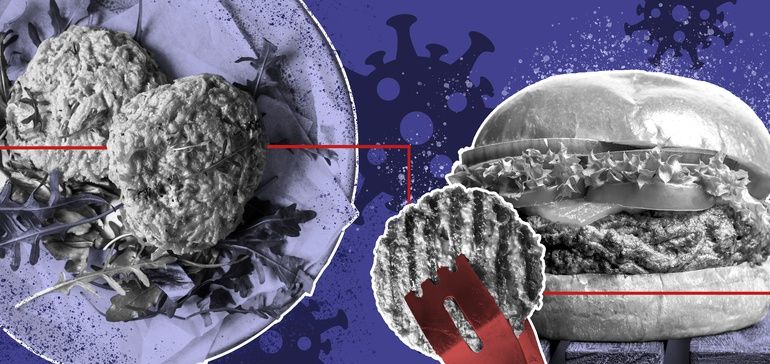
Sales for the category have exploded as the pandemic disrupts business as usual. Is this because people are turning away from animal-based eating, or is it keeping with the segment’s trends?
Category: biotech/medical
Over the past few decades, artificial intelligence (AI) tools have been used to analyze data or complete basic tasks in an increasing number of fields, ranging from computer science to manufacturing, medicine, physics, biology and even artistic disciplines. Researchers at University of Michigan have recently been investigating the use of artificial intelligence (AI) in architecture. Their most recent paper, published in the International Journal of Architectural Computing, specifically explores the potential of AI as a tool to create new architectural designs.
“My partner, Sandra Manninger, and myself, have a long-standing obsession with the idea to cross pollinate the fields of architecture and AI,” Matias del Campo, one of the researchers who carried out the study, told Tech Xplore. “We first got in touch with AI research in 1998, when we were introduced to the OFAI (The Austrian Institute of Artificial Intelligence) through a mutual friend, Dr. Arthur Flexer and we held the first course in Machine Learning for Architecture at the University of Applied Arts in Vienna, in 2006.”
Several years after they first became interested in the potential uses of AI in architecture, del Campo and Manninger started collaborating with the Robotics Department at University of Michigan. Working with Jessy Grizzle, the department’s director, and Alexandra Carlson, one of her Ph.D. students, they were able to significantly expand their research. Their study featured in the International Journal of Architectural Computing is the latest of a series of research efforts in which they investigated the use of AI techniques for designing architectural solutions.
Methylation definition at 5:05, 27:20 a lil about reprogramming, 32:00 q&a, 47:44 Aubrey chimes in, 57:00 Keith Comito(and other throughout)
Zoom transcription: https://otter.ai/u/AIIhn4i_p4DIXHAJx0ZaG0HUnAU
We will be joined by Morgan Levine, Yale University, to discuss the recent article “Underlying Features of Epigenetic Aging Clocks” she co-authored.
The talk will compare and contrast existing epigenetic clocks and describe how they can be deconstructed to facilitate our understanding of causes and consequences of epigenetic aging.
Article Abstract:
Epigenetic clocks, developed using DNA methylation data, have been widely used to quantify biological aging in multiple tissues/cells. However, many existing epigenetic clocks are weakly correlated with each other, suggesting they may capture different biological processes. We utilize multi‐omics data from diverse human tissue/cells to identify shared features across eleven existing epigenetic clocks. Despite the striking lack of overlap in CpGs, multi‐omics analysis suggested five clocks (Horvath1, Horvath2, Levine, Hannum, and Lin) share transcriptional associations conserved across purified CD14+ monocytes and dorsolateral prefrontal cortex. The pathways enriched in the shared transcriptional association suggested links between epigenetic aging and metabolism, immunity, and autophagy. Results from in vitro experiments showed that two clocks (Levine and Lin) were accelerated in accordance with two hallmarks of aging—cellular senescence and mitochondrial dysfunction. Finally, using multi‐tissue data to deconstruct the epigenetic clock signals, we developed a meta‐clock that demonstrated improved prediction for mortality and robustly related to hallmarks of aging in vitro than single clocks.
Morgan’s Bio:
Morgan Levine is a ladder-rank Assistant Professor in the Department of Pathology at the Yale School of Medicine and a member of both the Yale Combined Program in Computational Biology and Bioinformatics, and the Yale Center for Research on Aging. Her work relies on an interdisciplinary approach, integrating theories and methods from statistical genetics, computational biology, and mathematical demography to develop biomarkers of aging for humans and animal models using high-dimensional omics data. As PI or co-Investigator on multiple NIH-, Foundation-, and University-funded projects, she has extensive experience using systems-level and machine learning approaches to track epigenetic, transcriptomic, and proteomic changes with aging and incorporate this information to develop measures of risk stratification for major chronic diseases, such as cancer and Alzheimer’s disease. Her work also involves development of systems-level outcome measures of aging, aimed at facilitating evaluation for geroprotective interventions. A number of the existing biological aging measures she has developed are being applied in both basic and observational research.
AI, Genetics, and Health-Tech / Wearables — 21st Century Technologies For Healthy Companion Animals.
Ira Pastor ideaXme life sciences ambassador interviews Dr. Angela Hughes, the Global Scientific Advocacy Relations Senior Manager and Veterinary Geneticist at Mars Petcare.
The global petcare industry is significantly expanding, with North America sales alone expected to hit US $300 billion by 2025. And while we may associate the Mars Corporation, the world’s largest candy company, with leading confectionary brands like Milky Way, M&M’s, Skittles, Snickers, Twix, etc. They also happen to be one of the world’s largest companies in pet care as well.
Dr. Angela Hughes, is the Global Scientific Advocacy Relations Senior Manager & Veterinary Geneticist at Mars Petcare. Dr. Hughes is both Doctor of Veterinary Medicine, and a PhD with a focus in Canine Genetics, both from the University of California, Davis. Dr. Hughes also serves as Veterinary Genetics Research Manager of Wisdom Health, a business unit of Mars Petcare, which has developed state-of-the-art genetic tests for companion animals, leading to revolutionary personalized petcare. She also serves as a Veterinary Geneticist of Hughes Veterinary Consulting, focused on small animal and equine genetics and with a special interest in small animal reproduction and pediatrics.
Dr Hughes is published in multiple academic journals, including the Journal of the American Veterinary Medical Association and has contributed chapters for publication in Veterinary Clinics of North America Small Animal Practice: Pediatrics and Large Animal Internal Medicine.
On this ideaXme episode we will hear from Dr. Hughes about:
-Her background — how she developed an interest in veterinary medicine and animal genetics, and how she arrived at Mars Petcare.
-Her role as the senior manager of Global Scientific Advocacy Relations at Mars Petcare.
2020 episode of the CIUT program “Innovation Nation on Career Buzz” where I discussed the state of the Canadian aerospace industry is now online at the link below.
The last few minutes discusses the impact of Covid-19 on the aerospace industry. It’s not good although the interview is.
A recent study published Tuesday in the journal JAMA Internal Medicine found that most Americans are still susceptible to COVID-19.
According to the study, researchers studied the blood samples of 177,919 Americans across the nation, D.C., and Puerto Rico between July 27 and Sept. 24. They found that fewer than 10% of the people had detectable COVID antibodies.
“In this U.S. nationwide seroprevalence cross-sectional study, we found that as of September 2020, most persons in the US did not have detectable SARS-CoV-2 antibodies, and seroprevalence estimates varied widely by jurisdiction,” the authors concluded. “Continued biweekly testing of sera collected by commercial laboratories will allow for assessment of the changing epidemiology of SARS-CoV-2 in the U.S. in the coming months. Our results reinforce the need for continued public health preventive measures, including the use of face masks and social distancing, to limit the spread of SARS-CoV-2 in the U.S.”
Now those islands are some of the only remaining corners of the globe where the coronavirus doesn’t exist, thanks to their total suspension of inbound tourism and other nonessential travel.
These 8 countries are accepting American travelers for remote-work trips
The islands of Samoa, which include the U.S. territory of American Samoa, closed to nonessential travel in March and have not recorded any confirmed coronavirus cases. To enter, U.S. citizens must hold permanent residency and request permission from the Samoan Health Ministry to travel on a commercial flight to Samoa through Auckland, New Zealand, before quarantining for 14 days.
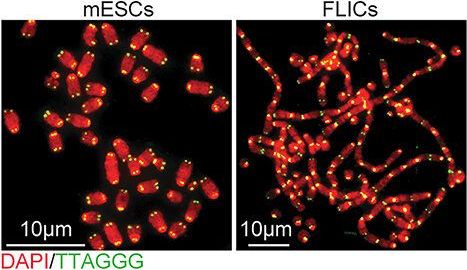
According to new research from CCR scientists, embryonic stem cells have a unique way of protecting their telomeres, the structures at the ends of chromosomes that shorten with every cell division. A research team led by Eros Lazzerini Denchi, Ph.D., an NIH Stadtman investigator in CCR’s Laboratory of Genomic Integrity, has found that rather than treating exposed telomeres as damaged DNA as most cells do, embryonic stem cells call on genes typically used only during the earliest stage of development to stave off unwanted DNA repair. The team’s findings, which come from studies of mouse embryonic stem cells, are reported November 25, 2020, in Nature.
By revealing an unexpected way cells can protect their telomeres, the new findings may help explain a survival strategy employed by some cancer cells, which must find a way to circumvent growth limits imposed by the natural shortening of telomeres that occurs as we age.
Embryonic stem cells, which arise early in an embryo’s development, have a unique capacity to become virtually any of the body’s specialized cell types. Lazzerini Denchi and colleagues first discovered their unusual approach to protecting telomeres when they found that the cells can survive without a protein called TRF2, which binds to and protects chromosome tips. The protein is absolutely essential for hundreds of different types of cells. Without it, exposed chromosome tips trigger faulty activation of DNA damage repair pathways, which stitch the unprotected ends together. Chromosomes fuse together and cells lose the ability to divide. But when Lazzerini Denchi’s team removed TRF2 from embryonic stem cells, chromosomes maintained their integrity and the cells continued to proliferate.