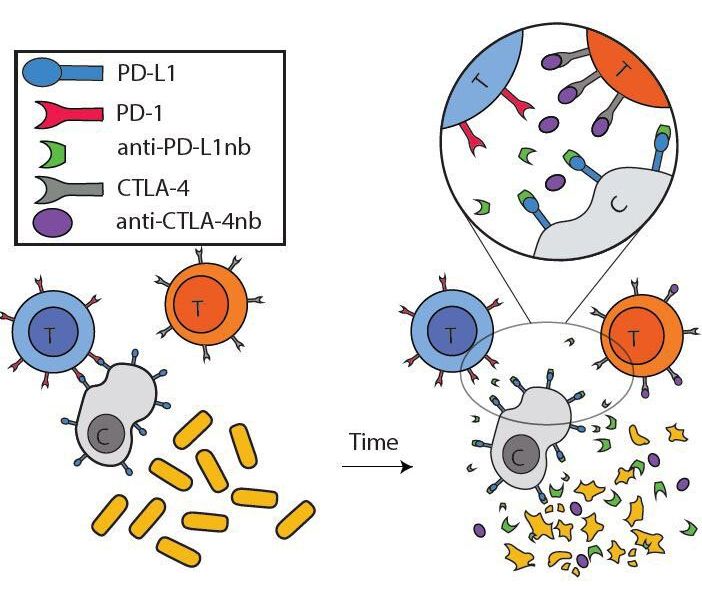
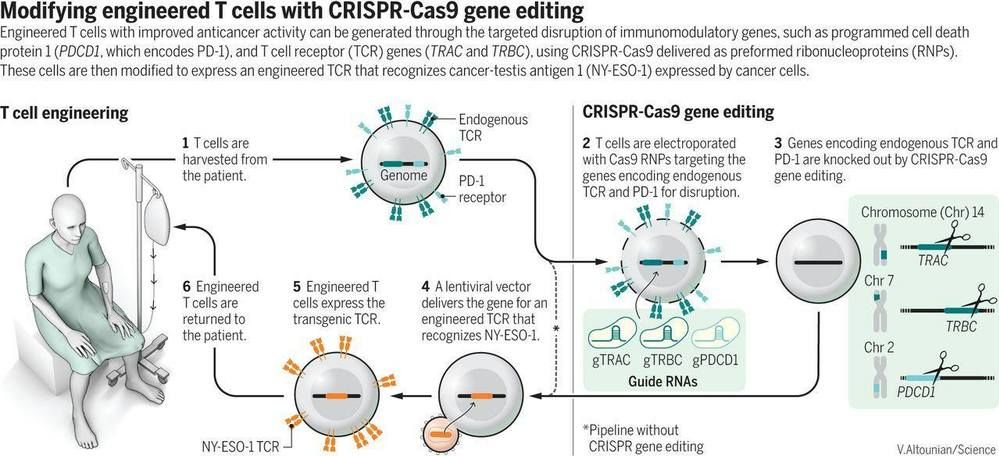
The generation of a chemical system capable of replication and evolution is a key objective of synthetic biology. This could be achieved by in vitro reconstitution of a minimal self-sustaining central dogma consisting of DNA replication, transcription and translation. Here, we present an in vitro translation system, which enables self-encoded replication and expression of large DNA genomes under well-defined, cell-free conditions. In particular, we demonstrate self-replication of a multipartite genome of more than 116 kb encompassing the full set of Escherichia coli translation factors, all three ribosomal RNAs, an energy regeneration system, as well as RNA and DNA polymerases. Parallel to DNA replication, our system enables synthesis of at least 30 encoded translation factors, half of which are expressed in amounts equal to or greater than their respective input levels. Our optimized cell-free expression platform could provide a chassis for the generation of a partially self-replicating in vitro translation system.