If you’re like me — you’re excited about the imminent increases to our healthspan that longevity technologies will soon offer us. However, if you want to stick around long enough to take advantage of all of the soon-to-be available lifespan and healthspan boosting technologies, you need to make sure you don’t die in the process!
How will you die? The four deadly killers
Ever since science effectively cured infectious disease through antibiotics, vaccinations and the like, there has been a distinct shift in what kills humans to the four deadly killers, which are considered ‘age related diseases’. These are — cardiovascular disease, neurodegenerative disease, metabolic disease and cancer. If you manage to escape the most likely causes of death as a young person, which are largely accidental accidental death (mostly car accidents), homicide or mental illness related (suicide) — then it is most likely that one of those four deadly killers will end your life.
But here’s the good news — there’s a growing body of immediately actionable longevity technologies that you can engage with to offset your risk of dying of these diseases. In a series of posts on the topic, I’m going to cover a few key resources at your disposal for minimising your risk for each of these four categories. First-up, cardiovascular disease.
Deadly Killer #1 — Cardiovascular disease
Heart attack, stroke, thrombosis, heart failure — the chances are overwhelming that you have lost someone important to you in your life to one of these causes. It is often seemingly sudden, but in most cases, the acute cause of death by cardiovascular disease has been brewing for a very long time — decades even.
The term ‘cardiovascular’ encompasses disease of both the heart and blood vessels, which is driven by the build up and eventual displacement of plaque that accumulated in the arterial wall in a process called ‘atherosclerosis’.
It’s not my role here to explain all of the mechanisms of this disease. Instead, I want to focus on four actionable tools you can work with your doctor to obtain access to, which will help you assess your risk profile and detect any elevated risk of an acute event (e.g. heart attack, stroke) at an early, treatable stage:
1. Test your ApoB (“A-PO-B”)
Stop using your LDL-C as your primary risk assessment tool (The “LDL” value too commonly called the “bad” cholesterol), and start tracking your ApoB. ApoB is a particular type of molecule attached to the types of lipoproteins carried by your LDL (and VLDL) that are the most likely to enter the arterial wall and lead to plaque formation. You need to know *how many* of these atherogenic particles you have present in a given volume of you blood — this drives your risk. Your ApoB value is influenced by diet and lifestyle and can be controlled with pharmaceutical intervention and possibly through certain forms of supplementation.
Learn more about ApoB at Healthline.
2. Do you have elevated Lpa (”L-P-little-A”)?
Lpa is another cardiovascular disease bad guy that may be in your bloodstream. Lipoprotein-a is a particle which carries cholesterol, fats and proteins and is made by your body, and how much of it you make is inherited. Elevated levels of Lpa increase your risk of a heart attack or stroke as they are known to cause atherosclerosis. You certainly need to know if you carry the genetic risk factor, and the earlier the better (i.e. get this test done as early as possible)! Levels of Lpa don’t change much over one’s lifetime, so testing it once is enough in most cases! Know your Lpa status, and better know your risk, and whether or not you should modify your diet, lifestyle and treatment options.
Learn more about Lpa from the lipoprotein-a foundation
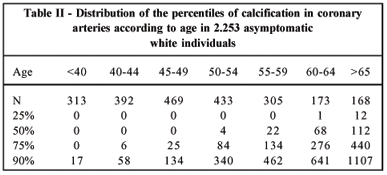
3. Know your Coronary Artery Calcium scan score
Coronary artery calcium (CAC) scans are created by using computed tomography (CT) scans, which are a type of X-ray scan, to detect the presence and quantity of coronary artery calcification (the warning signs of atherosclerosis).
A CAC test reveals both the location and quantity of calcium located in three of the main coronary arteries. The scan provides a score which represents your risk. The lower the better! This score will change over time, and is known to increase with age, so it is important to record it regularly (in a manner that balances the downsides of the X-ray radiation — ask your doctor what’s best for you). Atherosclerosis is a disease of ageing, and that means your risk is increasing over time. If you are aged 50 or above and have never had one — work with your doctor to get one performed.
4. Track your inflammation with C-Reactive Protein (CRP)
At its roots, atherosclerosis is known to be intimately connected with inflammation. In fact, it is often damage to the arterial wall that attracts the formation of plaque in the first place. This damage occurs over time, and is known to be increasingly likely with high blood pressure and high blood glucose levels. CRP is a very common and relatively low cost blood test that can be easily ordered up by your doctor, and should be tested annually at the very least. High levels of CRP are indicative of increased risk of cardiovascular disease, and once again can be influenced by changes in diet and exercise. If you have the option, go for the high-sensitivity CRP (hsCRP) test if possible!
Know thy risk, and save thy life!
Everything that I have discussed in this post encompasses longevity technologies that are available to you NOW. And ultimately, it is up to YOU to demand access to these technologies, in one way or another. I’d suggest that you don’t take NO for an answer, and that you allocate whatever resources (time, energy, money) you have available to assessing and managing your risk of cardiovascular disease. If you are aged 50 or older, the importance of getting each of these tests performed is exponentially more important with each decade of life!
I hope you enjoyed this post. I’ll be sure to come along with my follow-up posts on the topic of the four deadly killers in the coming weeks. In the meantime, check-out these other great Lifeboat Foundation blog posts on the topic of aging, and don’t forget to checkout the Longevity Blog on my website.
Wishing you a long and healthy life!
Dr Nick Engerer