They pack a powerful health wallop, but they’re tasty, too.


Wonderful to see the continuing progress of Mr. Omar Flores, with the support of his lovely wife, actress Mayra Sierra, today on the Venga la Alegria (VLA) show on TV Azteca (http://www.aztecauno.com/vengalaalegria) — The importance of an integrated approach to curing spinal cord injury including family, physical therapists, and the medical team at Regenerage (https://regenerage.clinic/)
https://www.youtube.com/watch?v=RRLo45i8q_A&t=4s


The cerebellum can’t get no respect. Located inconveniently on the underside of the brain and initially thought to be limited to controlling movement, the cerebellum has long been treated like an afterthought by researchers studying higher brain functions.
But researchers at Washington University School of Medicine in St. Louis say overlooking the cerebellum is a mistake. Their findings, published Oct. 25 in Neuron, suggest that the cerebellum has a hand in every aspect of higher brain functions — not just movement, but attention, thinking, planning and decision-making.
“The biggest surprise to me was the discovery that 80 percent of the cerebellum is devoted to the smart stuff,” said senior author Nico Dosenbach, MD, PhD, an assistant professor of neurology, of occupational therapy and of pediatrics. “Everyone thought the cerebellum was about movement. If your cerebellum is damaged, you can’t move smoothly — your hand jerks around when you try to reach for something. Our research strongly suggests that just as the cerebellum serves as a quality check on movement, it also checks your thoughts as well — smoothing them out, correcting them, perfecting things.”

To paraphrase Churchill’s words following the Second Battle of El Alamein: Google’s announcement about their new venture to extend human life, Calico, is not the end, nor even the beginning of the end, but it is, perhaps, the end of the beginning.
(MORE: Google vs. Death)
Since the dawn of civilization, humanity has been enslaved by the knowledge that no lifestyle choice, no medicine, no quirk of fate can enable anyone to live for more than a few decades without suffering progressive, inexorable decline in physical and mental function, leading inevitably to death. So soul-destroying has this knowledge been, for almost everyone, that we have constructed our entire society and world view around ways to put it out of our minds, mostly by convincing ourselves that the tragedy of aging is actually a good thing. And why not? After all, why be preoccupied about something one cannot affect?
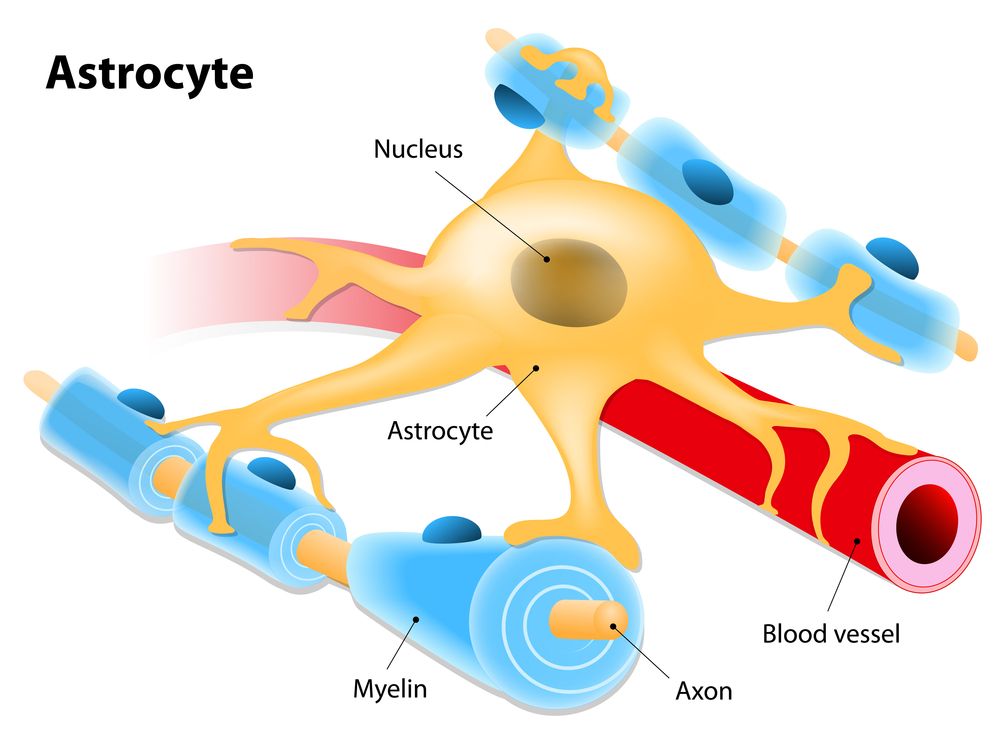
Protein Chrdl1 appears to regulate brain plasticity.
Researchers from the Salk Institute have discovered that a protein called Chrdl1, secreted by astrocytes, is responsible for driving synapse maturation and limiting brain plasticity later in life [1].
Abstract
In the developing brain, immature synapses contain calcium-permeable AMPA glutamate receptors (AMPARs) that are subsequently replaced with GluA2-containing calcium-impermeable AMPARs as synapses stabilize and mature. Here, we show that this essential switch in AMPARs and neuronal synapse maturation is regulated by astrocytes. Using biochemical fractionation of astrocyte-secreted proteins and mass spectrometry, we identified that astrocyte-secreted chordin-like 1 (Chrdl1) is necessary and sufficient to induce mature GluA2-containing synapses to form. This function of Chrdl1 is independent of its role as an antagonist of bone morphogenetic proteins (BMPs). Chrdl1 expression is restricted to cortical astrocytes in vivo, peaking at the time of the AMPAR switch. Chrdl1 knockout (KO) mice display reduced synaptic GluA2 AMPARs, altered kinetics of synaptic events, and enhanced remodeling in an in vivo plasticity assay.

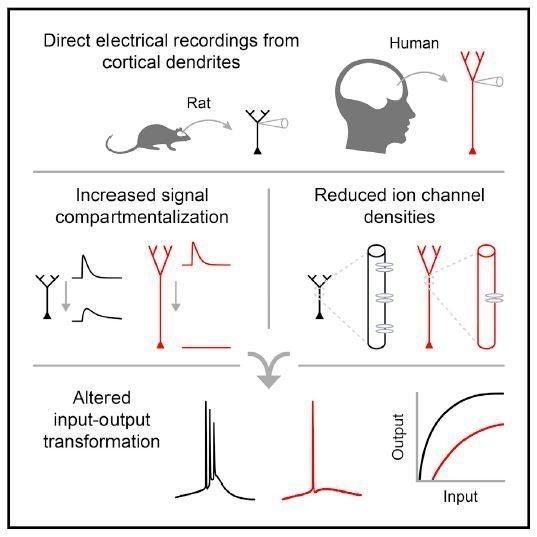
Human neurons are much larger than those of model organisms mice and rats, so it’s been unclear whether it’s size that makes a difference in our brain’s computational power. Now, in a study appearing October 18 in the journal Cell, researchers show that unlike those of other animals, human neurons employ highly compartmentalized signaling. Human dendrites—the tree-like branching structures that function as neurons’ antennas—process electrical signals differently than dendrites in rodents, the most common model systems for studying neuronal properties.
“The human neuron is basically like a rat neuron, but because it’s so much longer, signals have much farther to travel. The human dendrites thus have a different input-output function” from rats, says senior author Mark Harnett, the Fred and Carole Middleton Career Development Assistant Professor in the Department of Brain and Cognitive Sciences at the Massachusetts Institute of Technology. “Dendrites farther away from the cell body have fewer ion channels, which control signal processing. That was something we absolutely did not expect.”
Harnett, who studies how the biophysical features of neurons shape information processing in the brain, believes our longer, bigger dendritic arbors endow human neurons and their respective circuits with enhanced computational abilities.
Neuroscientists are debating the nature and meaning of neural activity during the critical moment of working memory when people must hold information in mind.
Scientists present dueling theories in the high-stakes quest to understand how we hold and juggle multiple pieces of information in mind.

Researchers healed mice with a genetic metabolic disorder that also affects humans by using a new editing tool to target and correct genetic mutations.
Some babies are born with the metabolic disorder phenylketonuria and need a special diet so that the amino acid phenylalanine doesn’t accumulate in the body. Excess phenylalanine delays mental and motor development. If left untreated, the children may develop mental disabilities.
The cause of this metabolic disorder is a mutation in a gene that provides the blueprint for the enzyme phenylalanine hydroxylase (Pah). The enzyme, which is produced by the cells of the liver, metabolizes phenylalanine.