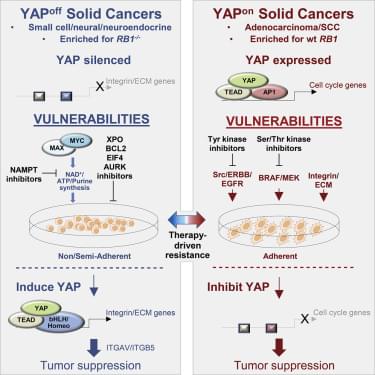
Cancer heterogeneity impacts therapeutic response, driving efforts to discover over-arching rules that supersede variability. Here, we define pan-cancer binary classes based on distinct expression of YAP and YAP-responsive adhesion regulators. Combining informatics with in vivo and in vitro gain-and loss-of-function studies across multiple murine and human tumor types, we show that opposite pro-or anti-cancer YAP activity functionally defines binary YAPon or YAPoff cancer classes that express or silence YAP, respectively. YAPoff solid cancers are neural/neuroendocrine and frequently RB1−/−, such as retinoblastoma, small cell lung cancer, and neuroendocrine prostate cancer. YAP silencing is intrinsic to the cell of origin, or acquired with lineage switching and drug resistance. The binary cancer groups exhibit distinct YAP-dependent adhesive behavior and pharmaceutical vulnerabilities, underscoring clinical relevance. Mechanistically, distinct YAP/TEAD enhancers in YAPoff or YAPon cancers deploy anti-cancer integrin or pro-cancer proliferative programs, respectively. YAP is thus pivotal across cancer, but in opposite ways, with therapeutic implications.
Pearson et al. demonstrate that YAP/TAZ, well-known oncogenes, are tumor suppressors in a large group of cancers. Pan-cancer analyses reveal that opposite YAP/TAZ expression, adhesive behavior, and oncogenic versus tumor suppressor YAP/TAZ activity functionally stratify binary cancer classes, which interchange to drive drug resistance. Contrasting YAPoff/YAPon classes exhibit unique vulnerabilities, facilitating therapeutic selection.