Startling discovery could open up avenues for treating some aggressive tumors.
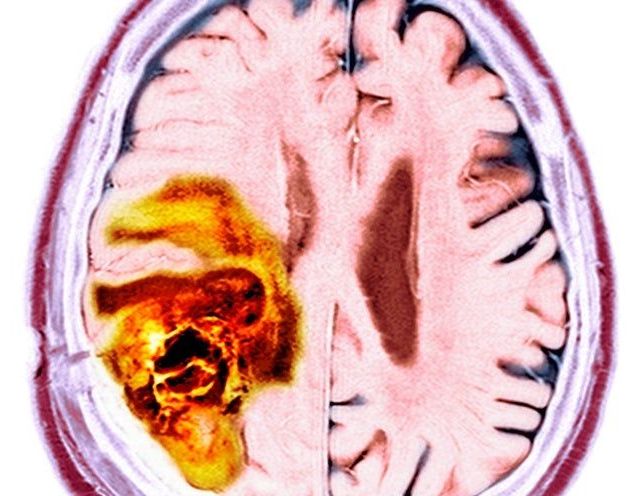

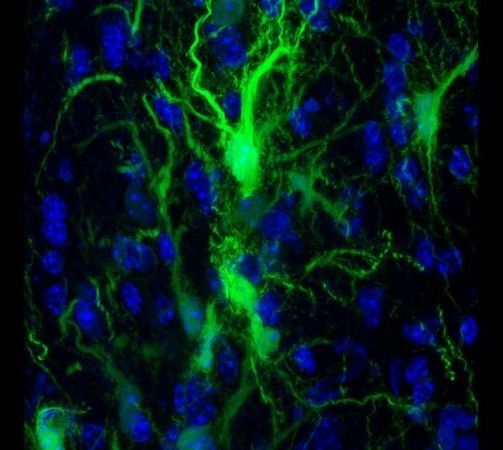

Cellular senescence, discovered in 1961 by Leonard Hayflick and Paul Moorhead, is a state in which cells no longer perform their functions, instead emitting harmful chemicals that turn other cells senescent. Senescence is primarily caused by telomere shortening and DNA damage, and senescent cells are known to contribute to multiple diseases, such as Alzheimer’s, Parkinson’s, and dementia.
One method of removing senescent cells is caloric restriction, which is a temporary reduction of food calories. This has been shown to be one of the most effective methods to decrease and slow the onset of aging phenotypes [1].
This is related to autophagy, which is the cell’s natural method of breaking down parts of itself when it doesn’t have immediate access to food [2]. Autophagy has been shown to both promote and prevent senescence. It removes damaged macromolecules or organelles, such as mitochondria, which would otherwise cause cellular senescence. However, some of the processes that cause autophagy cause cellular senescence as well [3].
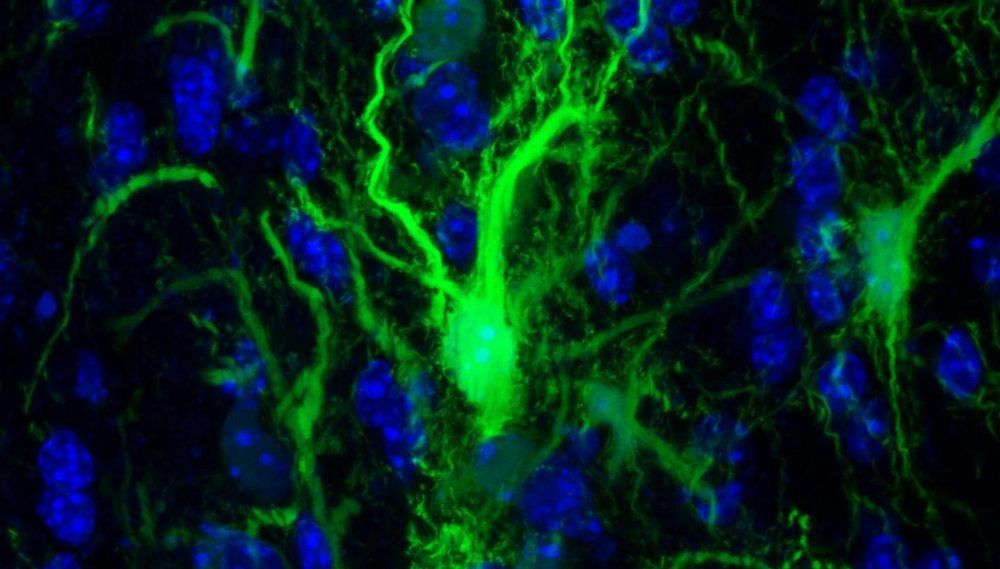
Transplanted brain stem cells survive without anti-rejection drugs in mice. By exploiting a feature of the immune system, researchers open the door for stem cell transplants to repair the brain.
In experiments in mice, Johns Hopkins Medicine researchers say they have developed a way to successfully transplant certain protective brain cells without the need for lifelong anti-rejection drugs.
A report on the research, published today (September 16, 2019) in the journal Brain, details the new approach, which selectively circumvents the immune response against foreign cells, allowing transplanted cells to survive, thrive and protect brain tissue long after stopping immune-suppressing drugs.
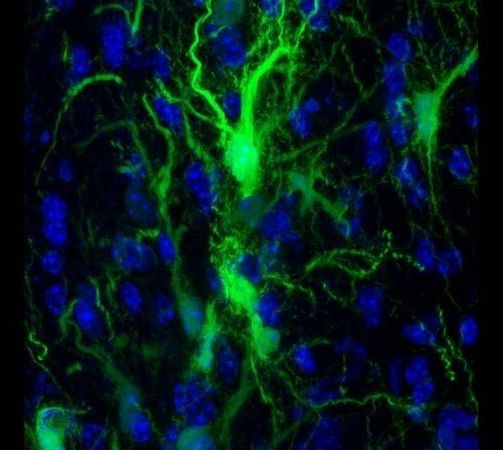
In experiments in mice, Johns Hopkins Medicine researchers say they have developed a way to successfully transplant certain protective brain cells without the need for lifelong anti-rejection drugs.
A report on the research, published Sept. 16 in the journal Brain, details the new approach, which selectively circumvents the immune response against foreign cells, allowing transplanted cells to survive, thrive and protect brain tissue long after stopping immune-suppressing drugs.
The ability to successfully transplant healthy cells into the brain without the need for conventional anti-rejection drugs could advance the search for therapies that help children born with a rare but devastating class of genetic diseases in which myelin, the protective coating around neurons that helps them send messages, does not form normally. Approximately 1 of every 100,000 children born in the U.S. will have one of these diseases, such as Pelizaeus-Merzbacher disease. This disorder is characterized by infants missing developmental milestones such as sitting and walking, having involuntary muscle spasms, and potentially experiencing partial paralysis of the arms and legs, all caused by a genetic mutation in the genes that form myelin.
Lab chemists and computer scientists are joining forces to find a nerve-agent antidote that will go where today s antidotes can t go the brain. Read more about in the latest issue of our Science & Technology Review magazine https://str.llnl.gov/2019-06/valdez

A new Royal Society report called “iHuman: blurring lines between mind and machine” is for the first time systematically exploring whether it is “right” or not to use neural interfaces – machines implanted in or worn over the body to pick up or stimulate nervous activity in the brain or other parts of the nervous system. It also sets out recommendations to ensure the ethical risks are understood, and to set up a transparent, public-driven but flexible regulatory framework which will allow the UK to lead innovative technology in this field.

Another magical flavonoid!
Researchers have created a compound, that when tested in mice, was able to promote the reconstruction of the myelin sheath surrounding neuronal axons. These findings could pave the way to a new treatment for combating demyelinating conditions such as multiple sclerosis (MS). The findings were published in Glia. “I think we’ll know in about a year if this is the exact right drug to try in human clinical trials,” explained senior study author Larry Sherman, Ph.D., in a recent press release.
“If it’s not, we know from the mouse studies that this approach can work. The question is, can this drug be adapted to bigger human brains?”
What is myelin?