http://spie.org/bios.
Boyden’s award-winning research has led to tools that can activate or silence neurons with light, enabling the causal assessment of how specific neurons contribute to normal and pathological brain functions.
Ed Boyden is the founder and principal investigator of the Synthetic Neurobiology Group at Massachusetts Institute of Technology (MIT). The group develops tools for controlling and observing the dynamic circuits of the brain, and uses these neurotechnologies to understand how cognition and emotion arise from brain network operation, as well as to enable systematic repair of intractable brain disorders such as epilepsy, Parkinson’s disease, post-traumatic stress disorder, and chronic pain.
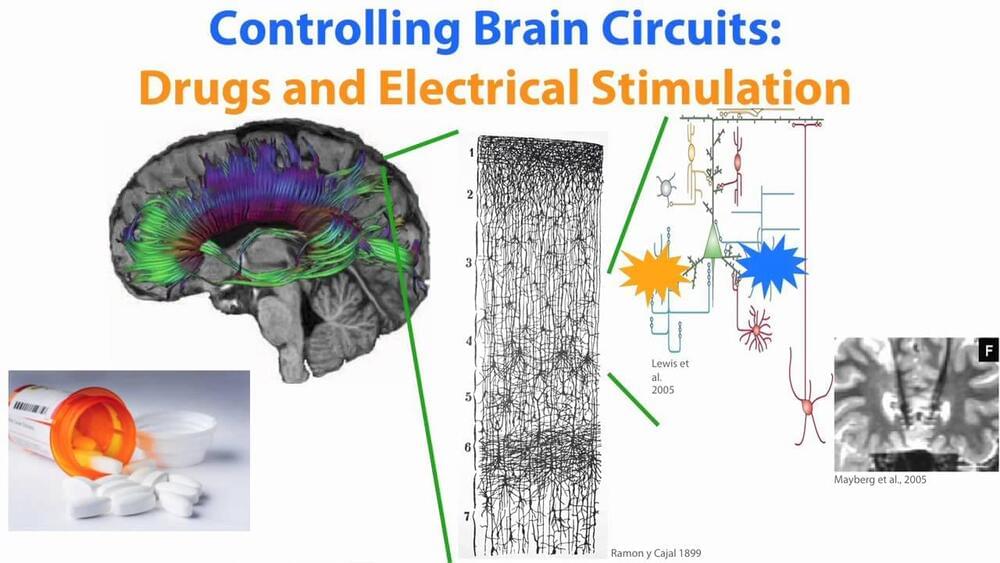
Many disorders of the brain currently are treated with drugs or electrical stimulation. Nearly a quarter of million people have implanted electrical probes in their brains for such stimulation. The problem with this approach is that it targets large areas of the brain instead of the discrete cells or location that cause the disorder. Boyden works on implementing light-stimulated processes in the brain to address these disorders at the cellular level. The method utilizes adeno-associated viruses (AAV) to create light-sensitive centers in the brain which can then be stimulated by light pulses. Very small optical waveguides (fibers) can then be introduced in the brain to stimulate these sites.
Boyden was named to the “Top 35 Innovators Under the Age of 35″ by Technology Review and to the “Top 20 Brains Under Age 40″ by Discover, and has received the NIH Director’s New Innovator Award, the Society for Neuroscience Research Award for Innovation in Neuroscience, and the Paul Allen Distinguished Investigator Award, as well as numerous other recognitions. In early 2011, he was an invited speaker at the renowned TED conference, sharing the bill with a high-powered lineup that included presenters as diverse as Bill Gates and choreographer Julie Taymor.
He has contributed numerous articles to SPIE Proceedings, and was an invited speaker at the Biomedical Optics Hot Topics Session at SPIE Photonics West 2011.